The NMDA receptor co-agonists, D-serine and glycine, regulate neuronal dendritic architecture in the somatosensory cortex
- PMID: 22024716
- PMCID: PMC3259183
- DOI: 10.1016/j.nbd.2011.10.006
The NMDA receptor co-agonists, D-serine and glycine, regulate neuronal dendritic architecture in the somatosensory cortex
Abstract
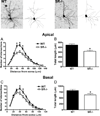
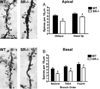
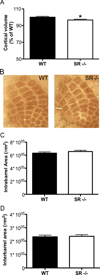
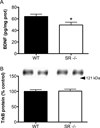
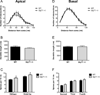
There is substantial evidence, both pharmacological and genetic, that hypofunction of the N-methyl-d-aspartate receptor (NMDAR) is a core pathophysiological feature of schizophrenia. There are morphological brain changes associated with schizophrenia, including perturbations in the dendritic morphology of cortical pyramidal neurons and reduction in cortical volume. Our experiments investigated whether these changes in dendritic morphology could be recapitulated in a genetic model of NMDAR hypofunction, the serine racemase knockout (SR-/-) mouse. Pyramidal neurons in primary somatosensory cortex (S1) of SR-/- mice had reductions in the complexity, total length, and spine density of apical and basal dendrites. In accordance with reduced cortical neuropil, SR-/- mice also had reduced cortical volume as compared to wild type mice. Analysis of S1 mRNA by DNA microarray and gene expression analysis revealed gene changes in SR-/- that are associated with psychiatric and neurologic disorders, as well as neurodevelopment. The microarray analysis also identified reduced expression of brain derived neurotrophic factor (BDNF) in SR-/- mice. Follow-up analysis by ELISA confirmed a reduction of BDNF protein levels in the S1 of SR-/- mice. Finally, S1 pyramidal neurons in glycine transporter heterozygote (GlyT1+/-) mutants, which display enhanced NMDAR function, had increased dendritic spine density. These results suggest that proper NMDAR function is important for the arborization and spine density of pyramidal neurons in cortex. Moreover, they suggest that NMDAR hypofunction might, in part, be contributing to the dendritic and synaptic changes observed in schizophrenia and highlight this signaling pathway as a potential target for therapeutic intervention.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

