Nicotinic acetylcholine receptors in dorsal root ganglion neurons include the α6β4* subtype
- PMID: 22024738
- PMCID: PMC3290440
- DOI: 10.1096/fj.11-195883
Nicotinic acetylcholine receptors in dorsal root ganglion neurons include the α6β4* subtype
Abstract
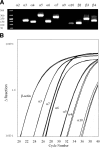
The α6-containing nicotinic acetylcholine receptors (nAChRs) have recently been implicated in diseases of the central nervous system (CNS), including Parkinson's disease and substance abuse. In contrast, little is known about the role of α6* nAChRs in the peripheral nervous system (where the asterisk denotes the possible presence of additional subunits). Dorsal root ganglia (DRG) neurons are known to express nAChRs with a pharmacology consistent with an α7, α3β4*, and α4β2* composition. Here we present evidence that DRG neurons also express α6* nAChRs. We used RT-PCR to show the presence of α6 subunit transcripts and patch-clamp electrophysiology together with subtype-selective α-conotoxins to pharmacologically characterize the nAChRs in rat DRG neurons. α-Conotoxin BuIA (500 nM) blocked acetylcholine-gated currents (I(ACh)) by 90.3 ± 3.0%; the recovery from blockade was very slow, indicating a predominance of α(x)β4* nAChRs. Perfusion with either 300 nM BuIA[T5A;P6O] or 200 nM MII[E11A], α-conotoxins that target the α6β4* subtype, blocked I(ACh) by 49.3 ± 5 and 46.7 ± 8%, respectively. In these neurons, I(ACh) was relatively insensitive to 200 nM ArIB[V11L;V16D] (9.4±2.0% blockade) or 500 nM PnIA (23.0±4% blockade), α-conotoxins that target α7 and α3β2*/α6β2* nAChRs, respectively. We conclude that α6β4* nAChRs are among the subtypes expressed by DRG, and to our knowledge, this is the first demonstration of α6β4* in neurons outside the CNS.
Figures
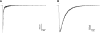

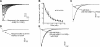
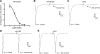
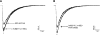

References
-
- Van Boxem K., van Bilsen J., de Meij N., Herrler A., Kessels F., Van Zundert J., van Kleef M. (2010) Pulsed radiofrequency treatment adjacent to the lumbar dorsal root ganglion for the management of lumbosacral radicular syndrome: a clinical audit. Pain Med. 12, 1322–1330 - PubMed
-
- Van Zundert J., Patijn J., Kessels A., Lame I., van Suijlekom H., van Kleef M. (2007) Pulsed radiofrequency adjacent to the cervical dorsal root ganglion in chronic cervical radicular pain: a double blind sham controlled randomized clinical trial. Pain 127, 173–182 - PubMed
-
- Simopoulos T. T., Kraemer J., Nagda J. V., Aner M., Bajwa Z. H. (2008) Response to pulsed and continuous radiofrequency lesioning of the dorsal root ganglion and segmental nerves in patients with chronic lumbar radicular pain. Pain Physician 11, 137–144 - PubMed
-
- Genzen J. R., Van Cleve W., McGehee D. S. (2001) Dorsal root ganglion neurons express multiple nicotinic acetylcholine receptor subtypes. J. Neurophysiol. 86, 1773–1782 - PubMed
-
- Dube G. R., Kohlhaas K. L., Rueter L. E., Surowy C. S., Meyer M. D., Briggs C. A. (2005) Loss of functional neuronal nicotinic receptors in dorsal root ganglion neurons in a rat model of neuropathic pain. Neurosci. Lett. 376, 29–34 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

