The induction of autophagy by mechanical stress
- PMID: 22024750
- PMCID: PMC3327616
- DOI: 10.4161/auto.7.12.17924
The induction of autophagy by mechanical stress
Abstract
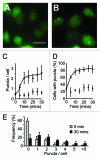
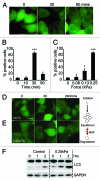
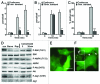
The ability to respond and adapt to changes in the physical environment is a universal and essential cellular property. Here we demonstrated that cells respond to mechanical compressive stress by rapidly inducing autophagosome formation. We measured this response in both Dictyostelium and mammalian cells, indicating that this is an evolutionarily conserved, general response to mechanical stress. In Dictyostelium, the number of autophagosomes increased 20-fold within 10 min of 1 kPa pressure being applied and a similar response was seen in mammalian cells after 30 min. We showed in both cell types that autophagy is highly sensitive to changes in mechanical pressure and the response is graduated, with half-maximal responses at ~0.2 kPa, similar to other mechano-sensitive responses. We further showed that the mechanical induction of autophagy is TOR-independent and transient, lasting until the cells adapt to their new environment and recover their shape. The autophagic response is therefore part of an integrated response to mechanical challenge, allowing cells to cope with a continuously changing physical environment.
Figures

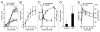

References
-
- Paglin S, Hollister T, Delohery T, Hackett N, McMahill M, Sphicas E, et al. A novel response of cancer cells to radiation involves autophagy and formation of acidic vesicles. Cancer Res. 2001;61:439–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
