Efficacy, safety, and pharmacokinetics of intravenous peramivir in children with 2009 pandemic H1N1 influenza A virus infection
- PMID: 22024821
- PMCID: PMC3256071
- DOI: 10.1128/AAC.00132-11
Efficacy, safety, and pharmacokinetics of intravenous peramivir in children with 2009 pandemic H1N1 influenza A virus infection
Abstract
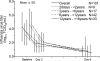
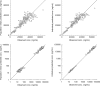
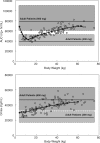
Peramivir is a new neuraminidase inhibitor for intravenous administration that was first introduced in clinical practice in Japan. We conducted a multicenter, open-label, uncontrolled study in children with influenza virus infection ranging in age from ≥28 days to <16 years during the 2009 pandemic A (H1N1) influenza epidemic to evaluate the efficacy, safety, and pharmacokinetics of peramivir in children after intravenous infusion of 10 mg/kg (600 mg maximum) once daily. Among the 106 children (125 days to 15 years old) confirmed to have been infected with the pH1N1 virus by the PCR who were treated with peramivir, the median time to alleviation of symptoms was 29.1 h (95% confidence interval = 22.1 to 32.4), and the proportion of the 106 children who were virus positive was 78.2% on day 2 after the start of treatment and had decreased to 7.1% on day 6. The results of the safety evaluation among 117 patients enrolled in this study showed that adverse events and adverse drug reactions were reported in 62.4 and 29.1%, respectively, of the patients. All of the adverse events and adverse drug reactions resolved or improved rapidly. A population pharmacokinetic analysis was performed on the basis of 297 observed plasma concentration data obtained from 115 children with influenza virus infection. Peramivir exposure in children was within the range of levels within which the efficacy and safety was confirmed in adults, and it is considered that peramivir is clinically and virologically effective and safe in children with pH1N1 virus infection.
Figures
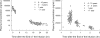
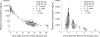
References
-
- Armitage P. 1955. Tests for linear trends in proportions and frequencies. Biometrics 11:375–386
-
- Bantia S, Arnold CS, Parker CD, Upshaw R, Chand P. 2006. Anti-influenza virus activity of peramivir in mice with single intramuscular injection. Antivir. Res. 69:39–45 - PubMed
-
- Birch MW. 1965. The detection of partial association II: the general case. J. R. Stat. Soc. Ser. B Stat. Methodol. 27:111–124
-
- Birnkrant D, Cox E. 2009. The emergency use authorization of peramivir for treatment of 2009 H1N1 influenza. N. Engl. J. Med. 361:2204–2207 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

