Pharmacokinetics of doxycycline in adults with cystic fibrosis
- PMID: 22024822
- PMCID: PMC3256044
- DOI: 10.1128/AAC.05710-11
Pharmacokinetics of doxycycline in adults with cystic fibrosis
Abstract
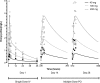
Cystic fibrosis (CF) is characterized by a chronic neutrophilic inflammatory response resulting in airway remodeling and progressive loss of lung function. Doxycycline is a tetracycline antibiotic that inhibits matrix metalloproteinase 9, a protease known to be associated with the severity of lung disease in CF. The pharmacokinetics of doxycycline was investigated during the course of a clinical trial to evaluate the short-term efficacy and safety in adults with CF. Plasma samples were obtained from 14 patients following a single intravenous dose and after 2 and 4 weeks of oral administration of doses ranging from 40 to 200 mg daily. The data were analyzed using noncompartmental and compartmental pharmacokinetics. The maximum concentration of drug in serum (C(max)) and area under the concentration-time curve from 0 h to infinity (AUC(0-∞)) values ranged from 1.0 to 3.16 mg/liter and 15.2 to 47.8 mg/liter × h, respectively, following single intravenous doses of 40 to 200 mg. C(max) and time to maximum concentration of drug in serum (T(max)) values following multiple-dose oral administration ranged from 1.15 to 3.04 mg/liter and 1.50 to 2.33 h, respectively, on day 14 and 1.48 to 3.57 mg/liter and 1.00 to 2.17 on day 28. Predose sputum/plasma concentration ratios on days 14 and 28 ranged from 0.33 to 1.1 (mean, 0.71 ± 0.33), indicating moderate pulmonary penetration. A 2-compartment model best described the combined intravenous and oral data. Absorption was slow and delayed (absorption rate constant [K(a)], 0.414 h(-1); lag time, 0.484 h) but complete (bioavailability [F], 1.16). The distribution and elimination half-lives were 0.557 and 18.1 h, respectively. Based on these data, the plasma concentrations at the highest dose, 200 mg/day, are in the range reported to produce anti-inflammatory effects in vivo and should be evaluated in clinical trials.
Figures
References
-
- Abdul-Hussien H, et al. 2009. Doxycycline therapy for abdominal aneurysm: improved proteolytic balance through reduced neutrophil content. J. Vasc. Surg. 49:741–749 - PubMed
-
- Baxter BT, et al. 2002. Prolonged administration of doxycycline in patients with small asymptomatic abdominal aortic aneurysms: report of a prospective (Phase II) multicenter study. J. Vasc. Surg. 36:1–12 - PubMed
-
- Brown DL, et al. 2004. Clinical and biochemical results of the metalloproteinase inhibition with subantimicrobial doses of doxycycline to prevent acute coronary syndromes (MIDAS) pilot trial. Arterioscler. Thromb. Vasc. Biol. 24:733–738 - PubMed
-
- Ciancio S, Ashley R. 1998. Safety and efficacy of sub-antimicrobial-dose doxycycline therapy in patients with adult periodontitis. Adv. Dent. Res. 12:27–31 - PubMed
-
- Fujita M, et al. 2007. Doxycycline attenuated lung injury by its biological effect apart from its antimicrobial function. Pulm. Pharmacol. Ther. 20:669–675 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

 ,…
,… 
