Transepithelial ion transport is suppressed in hypoxic sinonasal epithelium
- PMID: 22024847
- PMCID: PMC3205413
- DOI: 10.1002/lary.21921
Transepithelial ion transport is suppressed in hypoxic sinonasal epithelium
Abstract
Objectives/hypothesis: Sinonasal respiratory epithelial mucociliary clearance is dependent on the transepithelial transport of ions such as Cl(-) . The objectives of the present study were to investigate the role of oxygen restriction in 1) Cl(-) transport across primary sinonasal epithelial monolayers, 2) expression of the apical Cl(-) channels cystic fibrosis transmembrane conductance regulator (CFTR) and transmembrane protein 16A (TMEM16A), and 3) the pathogenesis of chronic rhinosinusitis.
Study design: In vitro investigation.
Methods: Murine nasal septal epithelial (MNSE), wild type, and human sinonasal epithelial (HSNE) cultures were incubated under hypoxic conditions (1% O(2) , 5% CO(2) ). Cultures were mounted in Ussing chambers for ion transport measurements. CFTR and TMEM16A expression were measured using quantitative reverse-transcription polymerase chain reaction (RT-PCR).
Results: The change in short-circuit current (ΔI(SC) in microamperes per square centimeter) attributable to CFTR (forskolin-stimulated) was significantly decreased due to a 12-hour hypoxia exposure in both MNSE (13.55 ± 0.46 vs. 19.23 ± 0.18) and HSNE (19.55 ± 0.56 vs. 25.49 ± 1.48 [control]; P < .05). TMEM16A (uridine triphosphate-stimulated transport) was inhibited by 48 hours of hypoxic exposure in MNSE (15.92 ± 2.87 vs. 51.44 ± 3.71 [control]; P < .05) and by 12 hours of hypoxic exposure in HSNE (16.75 ± 0.68 vs. 24.15 ± 1.35 [control]). Quantitative RT-PCR (reported as relative mRNA levels ± standard deviation) demonstrated significant reductions in both CFTR and TMEM16A mRNA expression in MNSE and HSNE owing to airway epithelial hypoxia.
Conclusions: Sinonasal epithelial CFTR and TMEM16A-mediated Cl(-) transport and mRNA expression were robustly decreased in an oxygen-restricted environment. These findings indicate that persistent hypoxia may lead to acquired defects in sinonasal Cl(-) transport in a fashion likely to confer mucociliary dysfunction in chronic rhinosinusitis.
Copyright © 2011 The American Laryngological, Rhinological, and Otological Society, Inc.
Conflict of interest statement
Conflict of Interest: Bradford Woodworth M.D. is a consultant for Arthrocare ENT and Gyrus ENT. Dr. Sorscher and Dr. Woodworth are inventors on a patent submitted regarding the possible activity of chloride secretagogues for therapy of sinus disease (Provisional Patent Application Under 35 U.S.C. ξ111(b) and 37 C.F.R ξ.53 (c) in the United States Patent and Trademark Office.
Figures





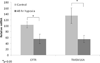
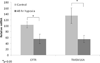


References
-
- Trout L, King M, Feng W, Inglis SK, Ballard ST. Inhibition of airway liquid secretion and its effect on the physical properties of airway mucus. Am J Physiol. 1998;274:L258–L263. - PubMed
-
- Anderson MP, Berger HA, Rich DP, Gregory RJ, Smith AE, Welsh MJ. Nucleoside triphosphates are required to open the CFTR chloride channel. Cell. 1991;67:775–784. - PubMed
-
- Caputo A, Caci E, Ferrera L, et al. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science. 2008;322:590–594. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

