Right ventricular outflow tract repair with a cardiac biologic scaffold
- PMID: 22025093
- PMCID: PMC3325605
- DOI: 10.1159/000331400
Right ventricular outflow tract repair with a cardiac biologic scaffold
Abstract
Background: Surgical reconstruction of congenital heart defects is often limited by the nonresorbable material used to approximate normal anatomy. In contrast, biologic scaffold materials composed of resorbable non-cross-linked extracellular matrix (ECM) have been used for tissue reconstruction of multiple organs and are replaced by host tissue. Preparation of whole organ ECM by decellularization through vascular perfusion can maintain much of the native three-dimensional (3D) structure, strength, and tissue-specific composition. A 3D cardiac ECM (C-ECM) biologic scaffold material would logically have structural and functional advantages over materials such as Dacron™ for myocardial repair, but the in vivo remodeling characteristics of C-ECM have not been investigated to date.
Methods and results: A porcine C-ECM patch or Dacron patch was used to reconstruct a full-thickness right ventricular outflow tract (RVOT) defect in a rat model with end points of structural remodeling function at 16 weeks. The Dacron patch was encapsulated by dense fibrous tissue and showed little cellular infiltration. Echocardiographic analysis showed that the right ventricle of the hearts patched with Dacron were dilated at 16 weeks compared to presurgery baseline values. The C-ECM patch remodeled into dense, cellular connective tissue with scattered small islands of cardiomyocytes. The hearts patched with C-ECM showed no difference in the size or function of the ventricles as compared to baseline values at both 4 and 16 weeks.
Conclusions: The C-ECM patch was associated with better functional and histomorphological outcomes compared to the Dacron patch in this rat model of RVOT reconstruction.
Copyright © 2011 S. Karger AG, Basel.
Figures







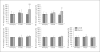
References
-
- Badylak S.F., Kochupura P.V., Cohen I.S., Doronin S.V., Saltman A.E., Gilbert T.W., Kelly D.J., Ignotz R.A., Gaudette G.R. The use of extracellular matrix as an inductive scaffold for the partial replacement of functional myocardium. Cell Transplant. 2006;15(suppl 1):S29–S40. - PubMed
-
- Badylak S.F., Kokini K., Tullius B., Simmons-Byrd A., Morff R. Morphologic study of small intestinal submucosa as a body wall repair device. J Surg Res. 2002;103:190–202. - PubMed
-
- Badylak S.F., Liang A., Record R., Tullius R., Hodde J.P. Endothelial cell adherence to small intestinal submucosa: an acellular bioscaffold. Biomaterials. 1999;20:2257–2263. - PubMed
-
- Badylak S.F., Park K., Peppas N., McCabe G., Yoder M. Marrow-derived cells populate scaffolds composed of xenogeneic extracellular matrix. Exp Hematol. 2001;29:1310–1318. - PubMed
-
- Badylak S.F., Vorp D.A., Spievack A.R., Simmons-Byrd A., Hanke J., Freytes D.O., Thapa A., Gilbert T.W., Nieponice A. Esophageal reconstruction with ECM and muscle tissue in a dog model. J Surg Res. 2005;128:87–97. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

