Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial
- PMID: 22025146
- PMCID: PMC3675689
- DOI: 10.1200/JCO.2011.35.5040
Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial
Erratum in
- J Clin Oncol. 2013 Aug 20;31(24):3049
Abstract
Purpose: There is no effective therapy for patients with advanced medullary thyroid carcinoma (MTC). Vandetanib, a once-daily oral inhibitor of RET kinase, vascular endothelial growth factor receptor, and epidermal growth factor receptor signaling, has previously shown antitumor activity in a phase II study of patients with advanced hereditary MTC.
Patients and methods: Patients with advanced MTC were randomly assigned in a 2:1 ratio to receive vandetanib 300 mg/d or placebo. On objective disease progression, patients could elect to receive open-label vandetanib. The primary end point was progression-free survival (PFS), determined by independent central Response Evaluation Criteria in Solid Tumors (RECIST) assessments.
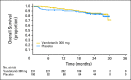
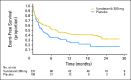
Results: Between December 2006 and November 2007, 331 patients (mean age, 52 years; 90% sporadic; 95% metastatic) were randomly assigned to receive vandetanib (231) or placebo (100). At data cutoff (July 2009; median follow-up, 24 months), 37% of patients had progressed and 15% had died. The study met its primary objective of PFS prolongation with vandetanib versus placebo (hazard ratio [HR], 0.46; 95% CI, 0.31 to 0.69; P < .001). Statistically significant advantages for vandetanib were also seen for objective response rate (P < .001), disease control rate (P = .001), and biochemical response (P < .001). Overall survival data were immature at data cutoff (HR, 0.89; 95% CI, 0.48 to 1.65). A final survival analysis will take place when 50% of the patients have died. Common adverse events (any grade) occurred more frequently with vandetanib compared with placebo, including diarrhea (56% v 26%), rash (45% v 11%), nausea (33% v 16%), hypertension (32% v 5%), and headache (26% v 9%).
Conclusion: Vandetanib demonstrated therapeutic efficacy in a phase III trial of patients with advanced MTC (ClinicalTrials.gov NCT00410761).
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

Comment in
-
Progress in molecular targeted therapy for thyroid cancer: vandetanib in medullary thyroid cancer.J Clin Oncol. 2012 Jan 10;30(2):119-21. doi: 10.1200/JCO.2011.37.8638. Epub 2011 Oct 24. J Clin Oncol. 2012. PMID: 22025155 No abstract available.
-
Pharmacotherapy: vandetanib-a new therapeutic option in advanced medullary thyroid cancer.Nat Rev Endocrinol. 2011 Nov 15;8(1):1. doi: 10.1038/nrendo.2011.198. Nat Rev Endocrinol. 2011. PMID: 22083086 No abstract available.
-
Drug therapy: a new standard for thyroid cancer?Nat Rev Clin Oncol. 2011 Nov 22;9(1):5. doi: 10.1038/nrclinonc.2011.174. Nat Rev Clin Oncol. 2011. PMID: 22104984 No abstract available.
-
Completing the Arc: targeted inhibition of RET in medullary thyroid cancer.J Clin Oncol. 2012 Jan 10;30(2):200-2. doi: 10.1200/JCO.2011.38.7639. Epub 2011 Dec 12. J Clin Oncol. 2012. PMID: 22162569 No abstract available.
-
Treatment of metastatic medullary thyroid cancer with vandetanib: need to stratify patients on basis of calcitonin doubling time.J Clin Oncol. 2012 Jun 10;30(17):2165; author reply 2166-7. doi: 10.1200/JCO.2012.42.3160. Epub 2012 May 7. J Clin Oncol. 2012. PMID: 22564994 No abstract available.
References
-
- Hundahl SA, Fleming ID, Fremgen AM, et al. A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985-1995. Cancer. 1998;83:2638–2648. see comments. - PubMed
-
- Lakhani VT, You YN, Wells SA. The multiple endocrine neoplasia syndromes. Annu Rev Med. 2007;58:253–265. - PubMed
-
- Roman S, Lin R, Sosa JA. Prognosis of medullary thyroid carcinoma: Demographic, clinical, and pathologic predictors of survival in 1252 cases. Cancer. 2006;107:2134–2142. - PubMed
-
- Modigliani E, Cohen R, Campos JM, et al. Prognostic factors for survival and for biochemical cure in medullary thyroid carcinoma: Results in 899 patients—The GETC Study Group, Groupe d'étude des tumeurs à calcitonine. Clin Endocrinol (Oxf) 1998;48:265–273. - PubMed
-
- Martins RG, Rajendran JG, Capell P, et al. Medullary thyroid cancer: Options for systemic therapy of metastatic disease? J Clin Oncol. 2006;24:1653–1655. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

