S0356: a phase II clinical and prospective molecular trial with oxaliplatin, fluorouracil, and external-beam radiation therapy before surgery for patients with esophageal adenocarcinoma
- PMID: 22025151
- PMCID: PMC3236655
- DOI: 10.1200/JCO.2011.36.7490
S0356: a phase II clinical and prospective molecular trial with oxaliplatin, fluorouracil, and external-beam radiation therapy before surgery for patients with esophageal adenocarcinoma
Abstract
Purpose: Pathologic complete response (pCR) after neoadjuvant therapy for locally advanced esophageal adenocarcinoma is associated with improved survival. The Southwest Oncology Group designed a trimodality, phase II, single-arm trial with objectives of achieving a pCR rate of 40% with prospective exploratory analyses of intratumoral molecular markers postulated to affect response and survival.
Patients and methods: Patients with clinically staged II or III esophageal adenocarcinoma received oxaliplatin 85 mg/m(2) on days 1, 15, and 29; protracted-infusion fluorouracil (PI-FU) 180 mg/m(2)/d on days 8 through 43; and external-beam radiation therapy (EBRT) 5 days a week at 1.8 Gy/d for 25 fractions; surgery was performed 28 to 42 days after neoadjuvant therapy. Chemotherapy was planned after surgery. Tumors were analyzed for mRNA expression and polymorphisms in genes involved in drug metabolism and DNA repair.
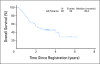
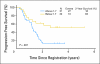
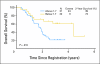
Results: Ninety-three patients were evaluable. Two deaths (2.2%) were attributable to preoperative therapy, and two deaths (2.2%) were attributable to surgery. Grade 3 and 4 toxicities were recorded for 47.3% and 19.4% of patients, respectively. Seventy-nine patients (84.9%) underwent surgery; 67.7% of patients had R0 resections. Twenty-six patients (28.0%) had confirmed pCR (95% CI, 19.1% to 38.2%). At a median follow-up of 39.2 months, estimates of median and 3-year overall survival (OS) were 28.3 months and 45.1%, respectively. Intratumoral ERCC-1 gene expression was inversely related to progression-free survival and OS.
Conclusion: Neoadjuvant oxaliplatin, PI-FU, and EBRT for esophageal adenocarcinoma is active and tolerable. Because the regimen failed to meet the primary end point, it does not define a new standard. However, future trials can be built on this platform to validate the role of ERCC-1 in determining the best systemic regimen for individual patients.
Trial registration: ClinicalTrials.gov NCT00086996.
Figures
References
-
- Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. - PubMed
-
- Gaast AV, van Hagen P, Hulshof M, et al. Effect of preoperative concurrent chemoradiotherapy on survival of patients with resectable esophageal or esophagogastric junction cancer: Results from a multicenter randomized phase III study. J Clin Oncol. 2010;28(suppl 15s):302s. abstr 4004.
-
- Suntharalingam M. Radiation therapy for esophageal cancer. Chest Surg Clin N Am. 2000;10:569–581. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U10 CA012644/CA/NCI NIH HHS/United States
- N01 CA032102/CA/NCI NIH HHS/United States
- U10 CA035192/CA/NCI NIH HHS/United States
- CA68183/CA/NCI NIH HHS/United States
- N01 CA045807/CA/NCI NIH HHS/United States
- N01 CA046441/CA/NCI NIH HHS/United States
- CA58882/CA/NCI NIH HHS/United States
- U10 CA035178/CA/NCI NIH HHS/United States
- U10 CA032102/CA/NCI NIH HHS/United States
- U10 CA046441/CA/NCI NIH HHS/United States
- CA-76448/CA/NCI NIH HHS/United States
- CA37981/CA/NCI NIH HHS/United States
- CA58416/CA/NCI NIH HHS/United States
- N01 CA035176/CA/NCI NIH HHS/United States
- CA35128/CA/NCI NIH HHS/United States
- U10 CA037981/CA/NCI NIH HHS/United States
- N01 CA035431/CA/NCI NIH HHS/United States
- U10 CA046113/CA/NCI NIH HHS/United States
- U10 CA045560/CA/NCI NIH HHS/United States
- CA12644/CA/NCI NIH HHS/United States
- U10 CA035128/CA/NCI NIH HHS/United States
- CA20319/CA/NCI NIH HHS/United States
- U10 CA045808/CA/NCI NIH HHS/United States
- U10 CA058416/CA/NCI NIH HHS/United States
- N01 CA035119/CA/NCI NIH HHS/United States
- CA45808/CA/NCI NIH HHS/United States
- CA58861/CA/NCI NIH HHS/United States
- CA35090/CA/NCI NIH HHS/United States
- CA46282/CA/NCI NIH HHS/United States
- U10 CA046282/CA/NCI NIH HHS/United States
- CA67663/CA/NCI NIH HHS/United States
- N01 CA035178/CA/NCI NIH HHS/United States
- N01 CA038926/CA/NCI NIH HHS/United States
- U10 CA067575/CA/NCI NIH HHS/United States
- CA-35176/CA/NCI NIH HHS/United States
- CA35192/CA/NCI NIH HHS/United States
- U10 CA058882/CA/NCI NIH HHS/United States
- U10 CA020319/CA/NCI NIH HHS/United States
- U10 CA038926/CA/NCI NIH HHS/United States
- U10 CA042777/CA/NCI NIH HHS/United States
- U10 CA035431/CA/NCI NIH HHS/United States
- CA-46113/CA/NCI NIH HHS/United States
- U10 CA035119/CA/NCI NIH HHS/United States
- CA42777/CA/NCI NIH HHS/United States
- CA52654/CA/NCI NIH HHS/United States
- U10 CA052654/CA/NCI NIH HHS/United States
- CA76429/CA/NCI NIH HHS/United States
- N01 CA067575/CA/NCI NIH HHS/United States
- U10 CA067663/CA/NCI NIH HHS/United States
- U10 CA035176/CA/NCI NIH HHS/United States
- U10 CA035090/CA/NCI NIH HHS/United States
- U10 CA058861/CA/NCI NIH HHS/United States
- U10 CA045807/CA/NCI NIH HHS/United States
- U10 CA068183/CA/NCI NIH HHS/United States
- N01 CA045560/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

