Cellular senescence and tumor suppressor gene p16
- PMID: 22025288
- PMCID: PMC3288293
- DOI: 10.1002/ijc.27316
Cellular senescence and tumor suppressor gene p16
Abstract
Cellular senescence is an irreversible arrest of cell growth. Biochemical and morphological changes occur during cellular senescence, including the formation of a unique cellular morphology such as flattened cytoplasm. Function of mitochondria, endoplasmic reticulum and lysosomes are affected resulting in the inhibition of lysosomal and proteosomal pathways. Cellular senescence can be triggered by a number of factors including, aging, DNA damage, oncogene activation and oxidative stress. While the molecular mechanism of senescence involves p16 and p53 tumor suppressor genes and telomere shortening, this review is focused on the mechanism of p16 control. The p16-mediated senescence acts through the retinoblastoma (Rb) pathway inhibiting the action of the cyclin dependant kinases leading to G1 cell cycle arrest. Rb is maintained in a hypophosphorylated state resulting in the inhibition of transcription factor E2F1. Regulation of p16 expression is complex and involves epigenetic control and multiple transcription factors. PRC1 (Pombe repressor complex (1) and PRC2 (Pombe repressor complex (2) proteins and histone deacetylases play an important role in the promoter hypermethylation for suppressing p16 expression. While transcription factors YY1 and Id1 suppress p16 expression, transcription factors CTCF, Sp1 and Ets family members activate p16 transcription. Senescence occurs with the inactivation of suppressor elements leading to the enhanced expression of p16.
Copyright © 2011 UICC.
Figures

 Activation
Activation  Inhibition
Inhibition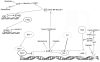
 Activation
Activation  Inhibition
Inhibition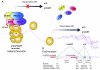
References
-
- Rabek JP, Boylston H., III Carbonylation of ER chaperone proteins in aged mouse liver. Biochem Biophys Res Commun. 2003;305:566–72. - PubMed
-
- Kornfeld R, Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–64. - PubMed
-
- Erickson RR, Dunning LM, Holtzman JL. The effect of aging on the chaperone concentrations in the hepatic, endoplasmic reticulum of male rats: the possible role of protein misfolding due to the loss of chaperones in the decline in physiological function seen with age. J Gerontol A Biol Sci Med Sci. 2006;61:435–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

