Dissociation of VE-PTP from VE-cadherin is required for leukocyte extravasation and for VEGF-induced vascular permeability in vivo
- PMID: 22025303
- PMCID: PMC3256962
- DOI: 10.1084/jem.20110525
Dissociation of VE-PTP from VE-cadherin is required for leukocyte extravasation and for VEGF-induced vascular permeability in vivo
Abstract
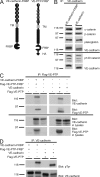
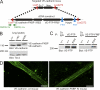
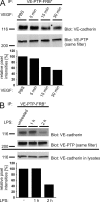
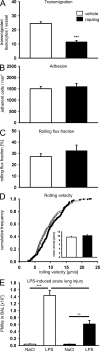
We have recently shown that vascular endothelial protein tyrosine phosphatase (VE-PTP), an endothelial membrane protein, associates with VE-cadherin and is required for optimal VE-cadherin function and endothelial cell contact integrity. The dissociation of VE-PTP from VE-cadherin is triggered by vascular endothelial growth factor (VEGF) and by the binding of leukocytes to endothelial cells in vitro, suggesting that this dissociation is a prerequisite for the destabilization of endothelial cell contacts. Here, we show that VE-cadherin/VE-PTP dissociation also occurs in vivo in response to LPS stimulation of the lung or systemic VEGF stimulation. To show that this dissociation is indeed necessary in vivo for leukocyte extravasation and VEGF-induced vascular permeability, we generated knock-in mice expressing the fusion proteins VE-cadherin-FK 506 binding protein and VE-PTP-FRB* under the control of the endogenous VE-cadherin promoter, thus replacing endogenous VE-cadherin. The additional domains in both fusion proteins allow the heterodimeric complex to be stabilized by a chemical compound (rapalog). We found that intravenous application of the rapalog strongly inhibited VEGF-induced (skin) and LPS-induced (lung) vascular permeability and inhibited neutrophil extravasation in the IL-1β inflamed cremaster and the LPS-inflamed lung. We conclude that the dissociation of VE-PTP from VE-cadherin is indeed required in vivo for the opening of endothelial cell contacts during induction of vascular permeability and leukocyte extravasation.
Figures
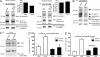
References
-
- Allingham M.J., van Buul J.D., Burridge K. 2007. ICAM-1-mediated, Src- and Pyk2-dependent vascular endothelial cadherin tyrosine phosphorylation is required for leukocyte transendothelial migration. J. Immunol. 179:4053–4064 - PubMed
-
- Bäumer S., Keller L., Holtmann A., Funke R., August B., Gamp A., Wolburg H., Wolburg-Buchholz K., Deutsch U., Vestweber D. 2006. Vascular endothelial cell-specific phosphotyrosine phosphatase (VE-PTP) activity is required for blood vessel development. Blood. 107:4754–4762 10.1182/blood-2006-01-0141 - DOI - PubMed
-
- Breviario F., Caveda L., Corada M., Martin-Padura I., Navarro P., Golay J., Introna M., Gulino D., Lampugnani M.G., Dejana E. 1995. Functional properties of human vascular endothelial cadherin (7B4/cadherin-5), an endothelium-specific cadherin. Arterioscler. Thromb. Vasc. Biol. 15:1229–1239 10.1161/01.ATV.15.8.1229 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

