A surface enolase participates in Borrelia burgdorferi-plasminogen interaction and contributes to pathogen survival within feeding ticks
- PMID: 22025510
- PMCID: PMC3255677
- DOI: 10.1128/IAI.05671-11
A surface enolase participates in Borrelia burgdorferi-plasminogen interaction and contributes to pathogen survival within feeding ticks
Abstract
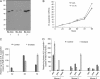
Borrelia burgdorferi, a tick-borne bacterial pathogen, causes a disseminated infection involving multiple organs known as Lyme disease. Surface proteins can directly participate in microbial virulence by facilitating pathogen dissemination via interaction with host factors. We show here that a fraction of the B. burgdorferi chromosomal gene product BB0337, annotated as enolase or phosphopyruvate dehydratase, is associated with spirochete outer membrane and is surface exposed. B. burgdorferi enolase, either in a recombinant form or as a membrane-bound native antigen, displays enzymatic activities intrinsic to the glycolytic pathway. However, the protein also interacts with host plasminogen, potentially leading to its activation and resulting in B. burgdorferi-induced fibrinolysis. As expected, enolase displayed consistent expression in vivo, however, with a variable temporal and spatial expression during spirochete infection in mice and ticks. Despite an extracellular exposure of the antigen and a potential role in host-pathogen interaction, active immunization of mice with recombinant enolase failed to evoke protective immunity against subsequent B. burgdorferi infection. In contrast, enolase immunization of murine hosts significantly reduced the acquisition of spirochetes by feeding ticks, suggesting that the protein could have a stage-specific role in B. burgdorferi survival in the feeding vector. Strategies to interfere with the function of surface enolase could contribute to the development of novel preventive measures to interrupt the spirochete infection cycle and reduce the incidences of Lyme disease.
Figures
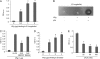





Similar articles
-
Borrelia burgdorferi and tick proteins supporting pathogen persistence in the vector.Future Microbiol. 2013 Jan;8(1):41-56. doi: 10.2217/fmb.12.121. Future Microbiol. 2013. PMID: 23252492 Free PMC article. Review.
-
The enolase of Borrelia burgdorferi is a plasminogen receptor released in outer membrane vesicles.Infect Immun. 2012 Jan;80(1):359-68. doi: 10.1128/IAI.05836-11. Epub 2011 Nov 14. Infect Immun. 2012. PMID: 22083700 Free PMC article.
-
The lipoprotein La7 contributes to Borrelia burgdorferi persistence in ticks and their transmission to naïve hosts.Microbes Infect. 2013 Sep-Oct;15(10-11):729-37. doi: 10.1016/j.micinf.2013.06.001. Epub 2013 Jun 15. Microbes Infect. 2013. PMID: 23774694 Free PMC article.
-
Borrelia burgdorferi enolase is a surface-exposed plasminogen binding protein.PLoS One. 2011;6(11):e27502. doi: 10.1371/journal.pone.0027502. Epub 2011 Nov 8. PLoS One. 2011. PMID: 22087329 Free PMC article.
-
Interaction of the Lyme disease spirochete with its tick vector.Cell Microbiol. 2016 Jul;18(7):919-27. doi: 10.1111/cmi.12609. Epub 2016 May 24. Cell Microbiol. 2016. PMID: 27147446 Free PMC article. Review.
Cited by
-
Borrelia burgdorferi and tick proteins supporting pathogen persistence in the vector.Future Microbiol. 2013 Jan;8(1):41-56. doi: 10.2217/fmb.12.121. Future Microbiol. 2013. PMID: 23252492 Free PMC article. Review.
-
Analysis of Paracoccidioides secreted proteins reveals fructose 1,6-bisphosphate aldolase as a plasminogen-binding protein.BMC Microbiol. 2015 Feb 27;15:53. doi: 10.1186/s12866-015-0393-9. BMC Microbiol. 2015. PMID: 25888027 Free PMC article.
-
Borrelia burgdorferi protein interactions critical for microbial persistence in mammals.Cell Microbiol. 2019 Feb;21(2):e12885. doi: 10.1111/cmi.12885. Epub 2018 Jul 8. Cell Microbiol. 2019. PMID: 29934966 Free PMC article. Review.
-
BB0347, from the lyme disease spirochete Borrelia burgdorferi, is surface exposed and interacts with the CS1 heparin-binding domain of human fibronectin.PLoS One. 2013 Sep 27;8(9):e75643. doi: 10.1371/journal.pone.0075643. eCollection 2013. PLoS One. 2013. PMID: 24086600 Free PMC article.
-
Delineating Surface Epitopes of Lyme Disease Pathogen Targeted by Highly Protective Antibodies of New Zealand White Rabbits.Infect Immun. 2019 Jul 23;87(8):e00246-19. doi: 10.1128/IAI.00246-19. Print 2019 Aug. Infect Immun. 2019. PMID: 31085705 Free PMC article.
References
-
- Agarwal S, Kulshreshtha P, Bambah Mukku D, Bhatnagar R. 2008. α-Enolase binds to human plasminogen on the surface of Bacillus anthracis. Biochim. Biophys. Acta 1784: 986–994 . - PubMed
-
- Barthold SW, Diego C, Philipp MT. 2010. Animal models of borreliosis, p 353–405 In Samuels DS, Radolf JD. (ed), Borrelia, molecular biology, host interaction and pathogenesis. Caister Academic Press, Norfolk, United Kingdom: .
-
- Bercic RL, et al. 2008. Identification of major immunogenic proteins of Mycoplasma synoviae isolates. Vet. Microbiol. 127: 147–154 . - PubMed
-
- Bergmann S, et al. 2003. Identification of a novel plasmin(ogen)-binding motif in surface displayed alpha-enolase of Streptococcus pneumoniae. Mol. Microbiol. 49: 411–423 . - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

