Alternative spermidine biosynthetic route is critical for growth of Campylobacter jejuni and is the dominant polyamine pathway in human gut microbiota
- PMID: 22025614
- PMCID: PMC3234850
- DOI: 10.1074/jbc.M111.307835
Alternative spermidine biosynthetic route is critical for growth of Campylobacter jejuni and is the dominant polyamine pathway in human gut microbiota
Abstract
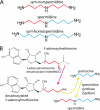
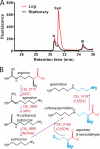
The availability of fully sequenced bacterial genomes has revealed that many species known to synthesize the polyamine spermidine lack the spermidine biosynthetic enzymes S-adenosylmethionine decarboxylase and spermidine synthase. We found that such species possess orthologues of the sym-norspermidine biosynthetic enzymes carboxynorspermidine dehydrogenase and carboxynorspermidine decarboxylase. By deleting these genes in the food-borne pathogen Campylobacter jejuni, we found that the carboxynorspermidine decarboxylase orthologue is responsible for synthesizing spermidine and not sym-norspermidine in vivo. In polyamine auxotrophic gene deletion strains of C. jejuni, growth is highly compromised but can be restored by exogenous sym-homospermidine and to a lesser extent by sym-norspermidine. The alternative spermidine biosynthetic pathway is present in many bacterial phyla and is the dominant spermidine route in the human gut, stomach, and oral microbiomes, and it appears to have supplanted the S-adenosylmethionine decarboxylase/spermidine synthase pathway in the gut microbiota. Approximately half of the gut Firmicutes species appear to be polyamine auxotrophs, but all encode the potABCD spermidine/putrescine transporter. Orthologues encoding carboxyspermidine dehydrogenase and carboxyspermidine decarboxylase are found clustered with an array of diverse putrescine biosynthetic genes in different bacterial genomes, consistent with a role in spermidine, rather than sym-norspermidine biosynthesis. Due to the pervasiveness of ε-proteobacteria in deep sea hydrothermal vents and to the ubiquity of the alternative spermidine biosynthetic pathway in that phylum, the carboxyspermidine route is also dominant in deep sea hydrothermal vents. The carboxyspermidine pathway for polyamine biosynthesis is found in diverse human pathogens, and this alternative spermidine biosynthetic route presents an attractive target for developing novel antimicrobial compounds.
Figures





Similar articles
-
Evolution and multifarious horizontal transfer of an alternative biosynthetic pathway for the alternative polyamine sym-homospermidine.J Biol Chem. 2010 May 7;285(19):14711-23. doi: 10.1074/jbc.M110.107219. Epub 2010 Mar 1. J Biol Chem. 2010. PMID: 20194510 Free PMC article.
-
Carboxyspermidine decarboxylase of the prominent intestinal microbiota species Bacteroides thetaiotaomicron is required for spermidine biosynthesis and contributes to normal growth.Amino Acids. 2016 Oct;48(10):2443-51. doi: 10.1007/s00726-016-2233-0. Epub 2016 Apr 27. Amino Acids. 2016. PMID: 27118128
-
An alternative polyamine biosynthetic pathway is widespread in bacteria and essential for biofilm formation in Vibrio cholerae.J Biol Chem. 2009 Apr 10;284(15):9899-907. doi: 10.1074/jbc.M900110200. Epub 2009 Feb 5. J Biol Chem. 2009. PMID: 19196710 Free PMC article.
-
Polyamine metabolism and cancer.J Cell Mol Med. 2003 Apr-Jun;7(2):113-26. doi: 10.1111/j.1582-4934.2003.tb00210.x. J Cell Mol Med. 2003. PMID: 12927050 Free PMC article. Review.
-
Polyamines.Annu Rev Biochem. 1984;53:749-90. doi: 10.1146/annurev.bi.53.070184.003533. Annu Rev Biochem. 1984. PMID: 6206782 Review. No abstract available.
Cited by
-
How a sugary bug gets through the day: recent developments in understanding fundamental processes impacting Campylobacter jejuni pathogenesis.Gut Microbes. 2012 Mar-Apr;3(2):135-44. doi: 10.4161/gmic.19488. Epub 2012 Mar 1. Gut Microbes. 2012. PMID: 22555465 Free PMC article. Review.
-
Polyamines and Gut Microbiota.Front Nutr. 2019 Feb 25;6:16. doi: 10.3389/fnut.2019.00016. eCollection 2019. Front Nutr. 2019. PMID: 30859104 Free PMC article. No abstract available.
-
Probiotics and their metabolite spermidine enhance IFN-γ+CD4+ T cell immunity to inhibit hepatitis B virus.Cell Rep Med. 2024 Nov 19;5(11):101822. doi: 10.1016/j.xcrm.2024.101822. Epub 2024 Nov 12. Cell Rep Med. 2024. PMID: 39536754 Free PMC article.
-
Polyamines in Eukaryotes, Bacteria, and Archaea.J Biol Chem. 2016 Jul 15;291(29):14896-903. doi: 10.1074/jbc.R116.734780. Epub 2016 Jun 7. J Biol Chem. 2016. PMID: 27268252 Free PMC article. Review.
-
A Narrative Review: Immunometabolic Interactions of Host-Gut Microbiota and Botanical Active Ingredients in Gastrointestinal Cancers.Int J Mol Sci. 2024 Aug 22;25(16):9096. doi: 10.3390/ijms25169096. Int J Mol Sci. 2024. PMID: 39201782 Free PMC article. Review.
References
-
- Pegg A. E. (2009) Essays Biochem. 46, 25–45 - PubMed
-
- Wu H., Min J., Ikeguchi Y., Zeng H., Dong A., Loppnau P., Pegg A. E., Plotnikov A. N. (2007) Biochemistry 46, 8331–8339 - PubMed
-
- Tabor C. W., Tabor H. (1976) Annu. Rev. Biochem. 45, 285–306 - PubMed
-
- Sekowska A., Coppée J. Y., Le Caer J. P., Martin-Verstraete I., Danchin A. (2000) Mol. Microbiol. 36, 1135–1147 - PubMed
-
- Toms A. V., Kinsland C., McCloskey D. E., Pegg A. E., Ealick S. E. (2004) J. Biol. Chem. 279, 33837–33846 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

