Dual role of junctin in the regulation of ryanodine receptors and calcium release in cardiac ventricular myocytes
- PMID: 22025663
- PMCID: PMC3286686
- DOI: 10.1113/jphysiol.2011.215988
Dual role of junctin in the regulation of ryanodine receptors and calcium release in cardiac ventricular myocytes
Abstract
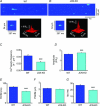
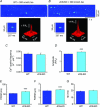
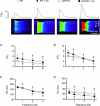
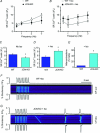
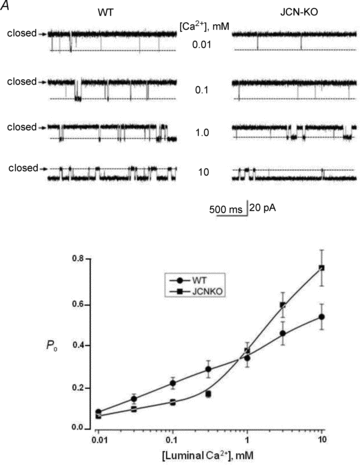
Junctin, a 26 kDa intra-sarcoplasmic reticulum (SR) protein, forms a quaternary complex with triadin, calsequestrin and the ryanodine receptor (RyR) at the junctional SR membrane. The physiological role for junctin in the luminal regulation of RyR Ca(2+) release remains unresolved, but it appears to be essential for proper cardiac function since ablation of junctin results in increased ventricular automaticity. Given that the junctin levels are severely reduced in human failing hearts, we performed an in-depth study of the mechanisms affecting intracellular Ca(2+) homeostasis in junctin-deficient cardiomyocytes. In concurrence with sparks, JCN-KO cardiomyocytes display increased Ca(2+) transient amplitude, resulting from increased SR [Ca(2+)] ([Ca(2+)](SR)). Junctin ablation appears to affect how RyRs 'sense' SR Ca(2+) load, resulting in decreased diastolic SR Ca(2+) leak despite an elevated [Ca(2+)](SR). Surprisingly, the β-adrenergic enhancement of [Ca(2+)](SR) reverses the decrease in RyR activity and leads to spontaneous Ca(2+) release, evidenced by the development of spontaneous aftercontractions. Single channel recordings of RyRs from WT and JCN-KO cardiac SR indicate that the absence of junctin produces a dual effect on the normally linear response of RyRs to luminal [Ca(2+)]: at low luminal [Ca(2+)] (<1 mmol l(-1)), junctin-devoid RyR channels are less responsive to luminal [Ca(2+)]; conversely, high luminal [Ca(2+)] turns them hypersensitive to this form of channel modulation. Thus, junctin produces complex effects on Ca(2+) sparks, transients, and leak, but the luminal [Ca(2+)]-dependent dual response of junctin-devoid RyRs demonstrates that junctin normally acts as an activator of RyR channels at low luminal [Ca(2+)], and as an inhibitor at high luminal [Ca(2+)]. Because the crossover occurs at a [Ca(2+)](SR) that is close to that present in resting cells, it is possible that the activator-inhibitor role of junctin may be exerted under periods of prevalent parasympathetic and sympathetic activity, respectively.
Figures


References
-
- Altschafl BA, Beutner G, Sharma VK, Sheu SS, Valdivia HH. The mitochondrial ryanodine receptor in rat heart: A pharmaco-kinetic profile. Biochim Biophys Acta. 2007;1768:1784–1795. - PubMed
-
- Benkusky NA, Weber CS, Scherman JA, Farrell EF, Hacker TA, Powers PA, Valdivia HH. Intact β-adrenergic response and unaltered progression towards heart failure in mice with genetic ablation of a major PKA phosphorylation site in the cardiac ryanodine receptor. Circ Res. 2007;101:819–829. - PubMed
-
- Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. - PubMed
-
- Cheng H, Lederer WJ. Calcium sparks. Physiol Rev. 2008;88:1491–1545. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

