Regulable neural progenitor-specific Tsc1 loss yields giant cells with organellar dysfunction in a model of tuberous sclerosis complex
- PMID: 22025691
- PMCID: PMC3214999
- DOI: 10.1073/pnas.1106454108
Regulable neural progenitor-specific Tsc1 loss yields giant cells with organellar dysfunction in a model of tuberous sclerosis complex
Abstract
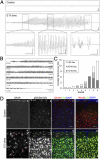
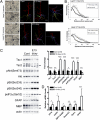
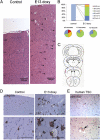
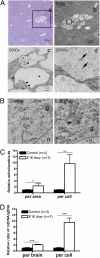
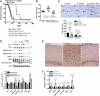
Tuberous sclerosis complex (TSC) is a multiorgan genetic disease in which brain involvement causes epilepsy, intellectual disability, and autism. The hallmark pathological finding in TSC is the cerebral cortical tuber and its unique constituent, giant cells. However, an animal model that replicates giant cells has not yet been described. Here, we report that mosaic induction of Tsc1 loss in neural progenitor cells in Tsc1(cc) Nestin-rtTA(+) TetOp-cre(+) embryos by doxycycline leads to multiple neurological symptoms, including severe epilepsy and premature death. Strikingly, Tsc1-null neural progenitor cells develop into highly enlarged giant cells with enlarged vacuoles. We found that the vacuolated giant cells had multiple signs of organelle dysfunction, including markedly increased mitochondria, aberrant lysosomes, and elevated cellular stress. We found similar vacuolated giant cells in human tuber specimens. Postnatal rapamycin treatment completely reversed these phenotypes and rescued the mutants from epilepsy and premature death, despite prenatal onset of Tsc1 loss and mTOR complex 1 activation in the developing brain. This TSC brain model provides insights into the pathogenesis and organelle dysfunction of giant cells, as well as epilepsy control in patients with TSC.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Jozwiak J, Jozwiak S, Wlodarski P. Possible mechanisms of disease development in tuberous sclerosis. Lancet Oncol. 2008;9(1):73–79. - PubMed
-
- Kwiatkowski DJ, Thiele EA, Whittemore VH, editors. Tuberous Sclerosis Complex: Genes, Clinical Features, and Therapeutics. Germany: Wiley–VCH, Weinheim; 2010. p. 432.
-
- Thiele EA. Managing epilepsy in tuberous sclerosis complex. J Child Neurol. 2004;19:680–686. - PubMed
-
- Holmes GL, Stafstrom CE, Tuberous Sclerosis Study Group Tuberous sclerosis complex and epilepsy: Recent developments and future challenges. Epilepsia. 2007;48:617–630. - PubMed
-
- Doherty C, Goh S, Young Poussaint T, Erdag N, Thiele EA. Prognostic significance of tuber count and location in tuberous sclerosis complex. J Child Neurol. 2005;20:837–841. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

