Discovery of β-arrestin-biased dopamine D2 ligands for probing signal transduction pathways essential for antipsychotic efficacy
- PMID: 22025698
- PMCID: PMC3215024
- DOI: 10.1073/pnas.1104807108
Discovery of β-arrestin-biased dopamine D2 ligands for probing signal transduction pathways essential for antipsychotic efficacy
Abstract
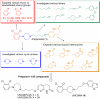
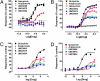
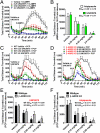
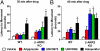
Elucidating the key signal transduction pathways essential for both antipsychotic efficacy and side-effect profiles is essential for developing safer and more effective therapies. Recent work has highlighted noncanonical modes of dopamine D(2) receptor (D(2)R) signaling via β-arrestins as being important for the therapeutic actions of both antipsychotic and antimanic agents. We thus sought to create unique D(2)R agonists that display signaling bias via β-arrestin-ergic signaling. Through a robust diversity-oriented modification of the scaffold represented by aripiprazole (1), we discovered UNC9975 (2), UNC0006 (3), and UNC9994 (4) as unprecedented β-arrestin-biased D(2)R ligands. These compounds also represent unprecedented β-arrestin-biased ligands for a G(i)-coupled G protein-coupled receptor (GPCR). Significantly, UNC9975, UNC0006, and UNC9994 are simultaneously antagonists of G(i)-regulated cAMP production and partial agonists for D(2)R/β-arrestin-2 interactions. Importantly, UNC9975 displayed potent antipsychotic-like activity without inducing motoric side effects in inbred C57BL/6 mice in vivo. Genetic deletion of β-arrestin-2 simultaneously attenuated the antipsychotic actions of UNC9975 and transformed it into a typical antipsychotic drug with a high propensity to induce catalepsy. Similarly, the antipsychotic-like activity displayed by UNC9994, an extremely β-arrestin-biased D(2)R agonist, in wild-type mice was completely abolished in β-arrestin-2 knockout mice. Taken together, our results suggest that β-arrestin signaling and recruitment can be simultaneously a significant contributor to antipsychotic efficacy and protective against motoric side effects. These functionally selective, β-arrestin-biased D(2)R ligands represent valuable chemical probes for further investigations of D(2)R signaling in health and disease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Luttrell LM, et al. Beta-arrestin-dependent formation of beta2 adrenergic receptor-Src protein kinase complexes. Science. 1999;283:655–661. - PubMed
-
- Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by beta-arrestins. Science. 2005;308:512–517. - PubMed
-
- Beaulieu JM, et al. An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122:261–273. - PubMed
-
- Urban JD, et al. Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther. 2007;320:1–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

