Noninvasive MRI of β-cell function using a Zn2+-responsive contrast agent
- PMID: 22025712
- PMCID: PMC3215051
- DOI: 10.1073/pnas.1109649108
Noninvasive MRI of β-cell function using a Zn2+-responsive contrast agent
Abstract
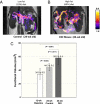
Elevation of postprandial glucose stimulates release of insulin from granules stored in pancreatic islet β-cells. We demonstrate here that divalent zinc ions coreleased with insulin from β-cells in response to high glucose are readily detected by MRI using the Zn(2+)-responsive T(1) agent, GdDOTA-diBPEN. Image contrast was significantly enhanced in the mouse pancreas after injection of a bolus of glucose followed by a low dose of the Zn(2+) sensor. Images of the pancreas were not enhanced by the agent in mice without addition of glucose to stimulate insulin release, nor were images enhanced in streptozotocin-treated mice with or without added glucose. These observations are consistent with MRI detection of Zn(2+) released from β-cells only during glucose-stimulated insulin secretion. Images of mice fed a high-fat (60%) diet over a 12-wk period and subjected to this same imaging protocol showed a larger volume of contrast-enhanced pancreatic tissue, consistent with the expansion of pancreatic β-cell mass during fat accumulation and progression to type 2 diabetes. This MRI sensor offers the exciting potential for deep-tissue monitoring of β-cell function in vivo during development of type 2 diabetes or after implantation of islets in type I diabetic patients.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Imaging Insulin Secretion from Mouse Pancreas by MRI Is Improved by Use of a Zinc-Responsive MRI Sensor with Lower Affinity for Zn2+ Ions.J Am Chem Soc. 2018 Dec 19;140(50):17456-17464. doi: 10.1021/jacs.8b07607. Epub 2018 Dec 11. J Am Chem Soc. 2018. PMID: 30484648 Free PMC article.
-
Imaging β-Cell Function Using a Zinc-Responsive MRI Contrast Agent May Identify First Responder Islets.Front Endocrinol (Lausanne). 2022 Jan 31;12:809867. doi: 10.3389/fendo.2021.809867. eCollection 2021. Front Endocrinol (Lausanne). 2022. PMID: 35173681 Free PMC article.
-
Dietary zinc reduction, pyruvate supplementation, or zinc transporter 5 knockout attenuates β-cell death in nonobese diabetic mice, islets, and insulinoma cells.J Nutr. 2012 Dec;142(12):2119-27. doi: 10.3945/jn.112.167031. Epub 2012 Oct 24. J Nutr. 2012. PMID: 23096014 Free PMC article.
-
Noninvasive assessment of pancreatic beta-cell function in vivo with manganese-enhanced magnetic resonance imaging.Am J Physiol Endocrinol Metab. 2009 Mar;296(3):E573-8. doi: 10.1152/ajpendo.90336.2008. Epub 2008 Dec 30. Am J Physiol Endocrinol Metab. 2009. PMID: 19116376 Free PMC article.
-
Zinc, a regulator of islet function and glucose homeostasis.Diabetes Obes Metab. 2009 Nov;11 Suppl 4:202-14. doi: 10.1111/j.1463-1326.2009.01110.x. Diabetes Obes Metab. 2009. PMID: 19817803 Review.
Cited by
-
Advances in using MRI probes and sensors for in vivo cell tracking as applied to regenerative medicine.Dis Model Mech. 2015 Apr;8(4):323-36. doi: 10.1242/dmm.018499. Epub 2015 Apr 1. Dis Model Mech. 2015. PMID: 26035841 Free PMC article. Review.
-
A Responsive Magnetic Resonance Imaging Contrast Agent for Detection of Excess Copper(II) in the Liver In Vivo.J Am Chem Soc. 2019 Jul 17;141(28):11009-11018. doi: 10.1021/jacs.8b13493. Epub 2019 Jul 3. J Am Chem Soc. 2019. PMID: 31268706 Free PMC article.
-
Zinc transporters and their role in the pancreatic β-cell.J Diabetes Investig. 2012 Jun 6;3(3):202-11. doi: 10.1111/j.2040-1124.2012.00199.x. J Diabetes Investig. 2012. PMID: 24843567 Free PMC article. Review.
-
Recent development of nanoparticles for molecular imaging.Philos Trans A Math Phys Eng Sci. 2017 Nov 28;375(2107):20170022. doi: 10.1098/rsta.2017.0022. Philos Trans A Math Phys Eng Sci. 2017. PMID: 29038377 Free PMC article. Review.
-
Dual-modal magnetic resonance/fluorescent zinc probes for pancreatic β-cell mass imaging.Chemistry. 2015 Mar 23;21(13):5023-33. doi: 10.1002/chem.201406008. Epub 2015 Mar 3. Chemistry. 2015. PMID: 25736590 Free PMC article.
References
-
- Scheen AJ. Diabetes mellitus in the elderly: Insulin resistance and/or impaired insulin secretion. Diabetes Metab. 2005;31:5S27–5S34. - PubMed
-
- Kim BJ, et al. Zinc as a paracrine effector in pancreatic islet cell death. Diabetes. 2000;49:367–372. - PubMed
-
- Wijesekara N, Chimienti F, Wheeler MB. Zinc, a regulator of islet function and glucose homeostasis. Diabetes Obes Metab. 2009;11(Suppl 4):202–214. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

