Replicative senescence of mesenchymal stem cells causes DNA-methylation changes which correlate with repressive histone marks
- PMID: 22025769
- PMCID: PMC3227452
- DOI: 10.18632/aging.100391
Replicative senescence of mesenchymal stem cells causes DNA-methylation changes which correlate with repressive histone marks
Abstract
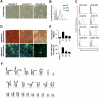
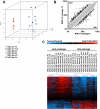
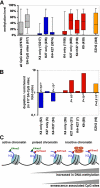
Cells in culture undergo replicative senescence. In this study, we analyzed functional, genetic and epigenetic sequels of long-term culture in human mesenchymal stem cells (MSC). Already within early passages the fibroblastoid colony-forming unit (CFU-f) frequency and the differentiation potential of MSC declined significantly. Relevant chromosomal aberrations were not detected by karyotyping and SNP-microarrays. Subsequently, we have compared DNA-methylation profiles with the Infinium HumanMethylation27 Bead Array and the profiles differed markedly in MSC derived from adipose tissue and bone marrow. Notably, all MSC revealed highly consistent senescence-associated modifications at specific CpG sites. These DNA-methylation changes correlated with histone marks of previously published data sets, such as trimethylation of H3K9, H3K27 and EZH2 targets. Taken together, culture expansion of MSC has profound functional implications - these are hardly reflected by genomic instability but they are associated with highly reproducible DNA-methylation changes which correlate with repressive histone marks. Therefore replicative senescence seems to be epigenetically controlled.
Conflict of interest statement
The authors of this manuscript have no conflict of interest to declare.
Figures


References
-
- Hayflick L., Moorhead P.S. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. - PubMed
-
- Greider C.W., Blackburn E.H. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell. 1985;43:405–413. - PubMed
-
- Di Donna S., Mamchaoui K., Cooper R.N., Seigneurin-Venin S., Tremblay J., Butler-Browne G.S., Mouly V. Telomerase can extend the proliferative capacity of human myoblasts, but does not lead to their immortalization. Mol Cancer Res. 2003;1:643–653. - PubMed
-
- Masutomi K., Yu E.Y., Khurts S., Ben-Porath I., Currier J.L., Metz G.B., Brooks M.W., Kaneko S., Murakami S., DeCaprio J.A., Weinberg R.A., Stewart S.A., Hahn W.C. Telomerase maintains telomere structure in normal human cells. Cell. 2003;114:241–253. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

