Antitubercular effect of 8-[(4-Chloro phenyl) sulfonyl]-7-Hydroxy-4-Methyl-2H-chromen-2-One in guinea pigs
- PMID: 22025853
- PMCID: PMC3198520
- DOI: 10.4103/0976-500X.85951
Antitubercular effect of 8-[(4-Chloro phenyl) sulfonyl]-7-Hydroxy-4-Methyl-2H-chromen-2-One in guinea pigs
Abstract
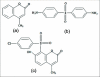
Objective: TO EVALUATE THE ANTITUBERCULAR EFFICACY AND SAFETY OF NEW CHEMICAL ENTITY (NCE): 8-[(4-Chloro phenyl) sulfonyl]-7-Hydroxy-4-Methyl-2H-chromen-2-One (CSHMC) in guinea pigs.
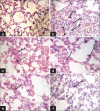
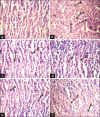
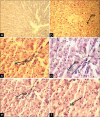
Materials and methods: This pilot study was carried out in guinea pigs. They were infected with M. tuberculosis H(37)Rv (1.5 × 10(4) cfu/guinea pig) via intramuscular route. After 30 days, infections were confirmed in 6 guinea pigs by histopathology of spleen, lung, and liver. After that CSHMC (5 and 20 mg/kg) was administered for 1 month and its effect was compared with vehicle, rifampicin (60 mg/kg) and isoniazid (30 mg/kg). Efficacy of CSHMC was evaluated on the basis of histopathologic scoring of lesion in lung, spleen, liver, and safety on the basis of measuring hemogram, liver and renal function parameters.
Results: Isoniazid, rifampicin, and CSHMC (20 mg/kg) significantly reduce the median lesion score in lung, spleen, and liver as compared to disease control group. Reduction in median lesion score for lung and spleen were not statistically significant for CSHMC 5 mg/kg. CSHMC (20 and 5 mg/kg) did not produce any changes in hemogram, liver and renal function parameters with respect to normal values.
Conclusions: CSHMC had shown significant antitubercular efficacy comparable to isoniazid and rifampicin and did not show hematological, hepato- and nephrotoxicity.
Keywords: Isoniazid; rifampicin; tuberculosis.
Conflict of interest statement
Figures
Similar articles
-
Poly (DL-lactide-co-glycolide) nanoparticle-based inhalable sustained drug delivery system for experimental tuberculosis.J Antimicrob Chemother. 2003 Dec;52(6):981-6. doi: 10.1093/jac/dkg477. Epub 2003 Nov 12. J Antimicrob Chemother. 2003. PMID: 14613962
-
[The animal experimental study on rifampicin-dependent Mycobacterium tuberculosis].Zhonghua Jie He He Hu Xi Za Zhi. 2006 Sep;29(9):617-21. Zhonghua Jie He He Hu Xi Za Zhi. 2006. PMID: 17129470 Chinese.
-
Comparison of the 'Denver regimen' against acute tuberculosis in the mouse and guinea pig.J Antimicrob Chemother. 2010 Apr;65(4):729-34. doi: 10.1093/jac/dkq007. Epub 2010 Jan 31. J Antimicrob Chemother. 2010. PMID: 20123722 Free PMC article.
-
Hepatotoxicity of antitubercular treatments. Rationale for monitoring liver status.Drug Saf. 1996 Dec;15(6):394-405. doi: 10.2165/00002018-199615060-00004. Drug Saf. 1996. PMID: 8968694 Review.
-
[Effectiveness and problems of PZA-containing 6-month regimen for the treatment of new pulmonary tuberculosis patients].Kekkaku. 2001 Jan;76(1):33-43. Kekkaku. 2001. PMID: 11211781 Review. Japanese.
Cited by
-
HigB1 Toxin in Mycobacterium tuberculosis Is Upregulated During Stress and Required to Establish Infection in Guinea Pigs.Front Microbiol. 2021 Nov 30;12:748890. doi: 10.3389/fmicb.2021.748890. eCollection 2021. Front Microbiol. 2021. PMID: 34917044 Free PMC article.
References
-
- Vasantha M, Gopi PG, Subramani R. Survival of tuberculosis patients treated under dots in a rural Tuberculosis unit (tu), south India. Indian J Tuberc. 2008;55:64–9. - PubMed
-
- Tomioka H, Namba K. Development of antituberculous drugs: Current status and future prospects. Kekkaku. 2006;81:753–74. - PubMed
-
- Dutt M, Khuller GK. Chemotherapy of Mycobacterium tuberculosis infections in mice with a combination of Isoniazide and Rifampicin entrapped in Poly (Dl-Lactide-Co-glycolide) microparticles. J Antimicrob Chemother. 2001;47:829–35. - PubMed
-
- Caminero JA. Management of multidrug-resistant tuberculosis and patients in retreatment. Eur Respir J. 2005;25:928–36. - PubMed
-
- Furin JJ, Johnson JL. Recent advances in the diagnosis and management of tuberculosis. Curr Opin Pulm Med. 2005;11:189–94. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
