A study of adverse drug reactions in pediatric patients
- PMID: 22025857
- PMCID: PMC3198524
- DOI: 10.4103/0976-500X.85957
A study of adverse drug reactions in pediatric patients
Abstract
Aim: To study the adverse drug reaction (ADR) pattern in a pediatric population in a tertiary care hospital.
Materials and methods: An observational study was done in the department of pediatrics in a tertiary care hospital. The ADRs occurring in the inpatient wards and outpatient department of pediatrics were actively monitored. The collected reports were analyzed for ADR pattern, drug groups, demographic profile, causality, severity, and preventability of the ADR.
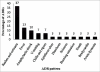
Results: A total of 30 ADRs were documented during the mid period of 2009 among pediatric patients. Most of the ADRs (60%) occurred below the age of 1 year. Antibiotics comprised the major group of drugs causing ADRs (67%). Rashes and urticaria were the most common type of ADR (37%) followed by fever, anaphylactic shock, vomiting, chills, and rigors. A single case of death had been reported in the study period. There were more occurrences of ADRs with multiple drugs compared to single drug therapy. About 80% of the ADRs were of probable causality and 87% were of probable preventability. There were no mild reactions, with 77% of reactions being moderate and 23% of reactions being severe in the severity scale.
Conclusions: ADRs occur more among infants and antibiotics were more commonly implicated. Most of the reactions were of moderate severity. This indicates the need for a rigid ADR monitoring among pediatric patients to ensure safety of drug therapy.
Keywords: Adverse drug reaction; causality; pediatric; pharmacovigilance.
Conflict of interest statement
Figures
References
-
- Le J, Nguyen T, Law AV, Hodding J. Adverse drug reactions among children over a 10-year period. Pediatrics. 2006;118:555–62. - PubMed
-
- Bavdekar SB, Karande S. National pharmacovigilance program. Indian Pediatr. 2006;43:27–32. - PubMed
-
- Clavenna A, Bonati M. Adverse drug reactions in childhood: A review of prospective studies and safety alerts. Arch Dis Child. 2009;94:724–8. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials