DANCING WITH THE ELECTRONS: TIME-DOMAIN AND CW IN VIVO EPR IMAGING
- PMID: 22025900
- PMCID: PMC3198805
- DOI: 10.4137/mri.s1131
DANCING WITH THE ELECTRONS: TIME-DOMAIN AND CW IN VIVO EPR IMAGING
Abstract
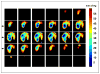
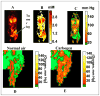
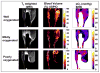
The progress in the development of imaging the distribution of unpaired electrons in living systems and the functional and the potential diagnostic dimensions of such an imaging process, using Electron Paramagnetic Resonance Imaging (EPRI), is traced from its origins with emphasis on our own work. The importance of EPR imaging stems from the fact that many paramagnetic probes show oxygen dependent spectral broadening. Assessment of in vivo oxygen concentration is an important factor in radiation oncology in treatment-planning and monitoring treatment-outcome. The emergence of narrow-line trairylmethyl based, bio-compatible spin probes has enabled the development of radiofrequency time-domain EPRI. Spectral information in time-domain EPRI can be achieved by generating a time sequence of T(2)* or T(2) weighted images. Progress in CW imaging has led to the use of rotating gradients, more recently rapid scan with direct detection, and a combination of all the three. Very low field MRI employing Dynamic Nuclear polarization (Overhauser effect) is also employed for monitoring tumor hypoxia, and re-oxygenation in vivo. We have also been working on the co-registration of MRI and time domain EPRI on mouse tumor models at 300 MHz using a specially designed resonator assembly. The mapping of the unpaired electron distribution and unraveling the spectral characteristics by using magnetic resonance in presence of stationary and rotating gradients in indeed 'dancing with the (unpaired) electrons', metaphorically speaking.
Figures













Similar articles
-
3-Carboxy-2,2,5,5-tetramethyl-pyrrolidinyl-N-oxyl.2008 Apr 30 [updated 2008 Jun 9]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2008 Apr 30 [updated 2008 Jun 9]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641530 Free Books & Documents. Review.
-
15N-Labeled 4-oxo-2,2,6,6-tetramethyl-piperidine-1-oxyl.2008 Apr 30 [updated 2008 Jun 9]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2008 Apr 30 [updated 2008 Jun 9]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641553 Free Books & Documents. Review.
-
3-Carbamoyl-2,2,5,5-tetramethyl-1-pyrrolidinyl-N-oxyl.2008 Apr 30 [updated 2008 Jun 9]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2008 Apr 30 [updated 2008 Jun 9]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641525 Free Books & Documents. Review.
-
In vivo imaging of a stable paramagnetic probe by pulsed-radiofrequency electron paramagnetic resonance spectroscopy.Magn Reson Med. 1997 Sep;38(3):409-14. doi: 10.1002/mrm.1910380309. Magn Reson Med. 1997. PMID: 9339442
-
EPR and Related Magnetic Resonance Imaging Techniques in Cancer Research.Metabolites. 2023 Jan 1;13(1):69. doi: 10.3390/metabo13010069. Metabolites. 2023. PMID: 36676994 Free PMC article. Review.
Cited by
-
Digital detection and processing of multiple quadrature harmonics for EPR spectroscopy.J Magn Reson. 2010 Dec;207(2):322-31. doi: 10.1016/j.jmr.2010.09.016. Epub 2010 Sep 29. J Magn Reson. 2010. PMID: 20971667 Free PMC article.
-
ESR Microscopy for Biological and Biomedical Applications.Nanosci Nanotechnol Lett. 2011 Aug;3(4):561-567. doi: 10.1166/nnl.2011.1206. Nanosci Nanotechnol Lett. 2011. PMID: 21984955 Free PMC article.
-
Development of a fast-scan EPR imaging system for highly accelerated free radical imaging.Magn Reson Med. 2019 Aug;82(2):842-853. doi: 10.1002/mrm.27759. Epub 2019 Apr 25. Magn Reson Med. 2019. PMID: 31020713 Free PMC article.
-
Optimization of magnetic field sweep and field modulation amplitude for continuous-wave EPR oximetry.J Magn Reson. 2011 Apr;209(2):337-40. doi: 10.1016/j.jmr.2011.01.013. Epub 2011 Jan 26. J Magn Reson. 2011. PMID: 21334232 Free PMC article.
-
Hypoxia Imaging As a Guide for Hypoxia-Modulated and Hypoxia-Activated Therapy.Antioxid Redox Signal. 2022 Jan;36(1-3):144-159. doi: 10.1089/ars.2021.0176. Antioxid Redox Signal. 2022. PMID: 34428981 Free PMC article. Review.
References
-
- Alecci M, Dellapenna S, Sotgui A, et al. Electron-Paramagnetic Resonance Spectrometer for 3-Dimensional Invivo Imaging at Very Low-Frequency. Rev Sci Instrum. 1992;63:4263–4270.
-
- Alecci M, Brivati JA, Placidi G, et al. A submicrosecond resonator and receiver system for pulsed magnetic resonance with large samples. J Magn Reson. 1998;132:162–166.
-
- Ardenkjaer-Larsen JH, Laursen I, Leunbach I, et al. EPR and DNP properties of certain novel single electron contrast agents intended for oximetric imaging. J Magn Reson. 1998;133:1–12. - PubMed
-
- Balcom B, Beyea SD, Green DP, et al. Single-Point Ramped Imaging with T1 Enhancement (SPRITE) J Magn Reson A. 1996a;123:131–134. - PubMed
-
- Balcom SD, Beyea SD, Green DP, et al. Imaging of heterogeneous materials with a turbo spin echo single-point imaging technique. J Magn Reson. 1996b;144:255–265. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

