Dual-compartment neurofluidic system for electrophysiological measurements in physically segregated and functionally connected neuronal cell culture
- PMID: 22025913
- PMCID: PMC3198030
- DOI: 10.3389/fneng.2011.00013
Dual-compartment neurofluidic system for electrophysiological measurements in physically segregated and functionally connected neuronal cell culture
Abstract
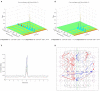
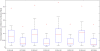
We developed a dual-compartment neurofluidic system with inter-connecting microchannels to connect neurons from their respective compartments, placed on a planar microelectrode arrays. The design and development of the compartmented microfluidic device for neuronal cell culture, protocol for sustaining long-term cultures, and neurite growth through microchannels in such a closed compartment device are presented. Using electrophysiological measurements of spontaneous network activity in the compartments and selective pharmacological manipulation of cells in one compartment, the biological origin of network activity and the fluidic isolation between the compartments are demonstrated. The connectivity between neuronal populations via the microchannels and the crossing-over of neurites are verified using transfection experiments and immunofluorescence staining. In addition to the neurite cross-over to the adjacent compartment, functional connectivity between cells in both the compartments is verified using cross-correlation (CC) based techniques. Bidirectional signal propagation between the compartments is demonstrated using functional connectivity maps. CC analysis and connectivity maps demonstrate that the two neuronal populations are not only functionally connected within each compartment but also with each other and a well connected functional network was formed between the compartments despite the physical barrier introduced by the microchannels.
Keywords: compartmented device; functional connectivity; invitro model; neuron cell culture; pharmacological manipulation.
Figures




References
-
- Berdondini L., Chippalone M., Van Der Wal P. D., Imfeld K., De Rooij N. F., Koudelka-Hep M., Tedesco M., Martinoia S., Van Pelt J., Le Masson G. (2006). A microelectrode array (MEA) integrated with clustering structures for investigating in vitro neurodynamics in confined interconnected sub-populations of neurons. Sen. Actuators B Chem. 114, 530–54110.1016/j.snb.2005.04.042 - DOI
-
- Berdondini L., Massobrio P., Chiappalone M., Tedesco M., Imfeld K., Maccione A., Gandolfo M., Koudelka-Hep M., Martinoia S. (2009). Extracellular recordings from locally dense microelectrode arrays coupled to dissociated cortical cultures. J. Neurosci. Methods 177, 386–39610.1016/j.jneumeth.2008.10.032 - DOI - PubMed
-
- Bodas D., Khan-Malek C. (2007). Hydrophilization and hydrophobic recovery of PDMS by oxygen plasma and chemical treatment – An SEM investigation. Sens. Actuators B Chem. 123, 368–37310.1016/j.snb.2006.08.037 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

