Canadian Optically-guided approach for Oral Lesions Surgical (COOLS) trial: study protocol for a randomized controlled trial
- PMID: 22026481
- PMCID: PMC3226575
- DOI: 10.1186/1471-2407-11-462
Canadian Optically-guided approach for Oral Lesions Surgical (COOLS) trial: study protocol for a randomized controlled trial
Abstract
Background: Oral cancer is a major health problem worldwide. The 5-year survival rate ranges from 30-60%, and has remained unchanged in the past few decades. This is mainly due to late diagnosis and high recurrence of the disease. Of the patients who receive treatment, up to one third suffer from a recurrence or a second primary tumor. It is apparent that one major cause of disease recurrence is clinically unrecognized field changes which extend beyond the visible tumor boundary. We have previously developed an approach using fluorescence visualization (FV) technology to improve the recognition of the field at risk surrounding a visible oral cancer that needs to be removed and preliminary results have shown a significant reduction in recurrence rates.
Method/design: This paper describes the study design of a randomized, multi-centre, double blind, controlled surgical trial, the COOLS trial. Nine institutions across Canada will recruit a total of 400 patients with oral severe dysplasia or carcinoma in situ (N = 160) and invasive squamous cell carcinoma (N = 240). Patients will be stratified by participating institution and histology grade and randomized equally into FV-guided surgery (experimental arm) or white light-guided surgery (control arm). The primary endpoint is a composite of recurrence at or 1 cm within the previous surgery site with 1) the same or higher grade histology compared to the initial diagnosis (i.e., the diagnosis used for randomization); or 2) further treatment due to the presence of severe dysplasia or higher degree of change at follow-up. This is the first randomized, multi-centre trial to validate the effectiveness of the FV-guided surgery.
Discussion: In this paper we described the strategies, novelty, and challenges of this unique trial involving a surgical approach guided by the FV technology. The success of the trial requires training, coordination, and quality assurance across multiple sites within Canada. The COOLS trial, an example of translational research, may result in reduced recurrence rates following surgical treatment of early-stage oral cancer with significant impacts on survival, morbidity, patients' quality of life and the cost to the health care system.
Trial registration: Clinicaltrials.gov NCT01039298.
Figures

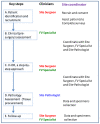
References
-
- Ferlay J, Bray F, Pisani P, Parkin D. IARC Cancer Base No. 5 Version 2.0. Lyon, France: IARCPress; 2004. GLOBOCAN 2002. Cancer incidence, mortality and prevalence worldwide.
-
- Braakhuis BJ, Tabor MP, Kummer JA, Leemans CR, Brakenhoff RH. A genetic explanation of Slaughter's concept of field cancerization: evidence and clinical implications. Cancer Res. 2003;63(8):1727–1730. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

