Design properties of hydrogel tissue-engineering scaffolds
- PMID: 22026626
- PMCID: PMC3206299
- DOI: 10.1586/erd.11.27
Design properties of hydrogel tissue-engineering scaffolds
Abstract
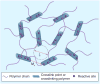
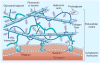
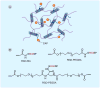
This article summarizes the recent progress in the design and synthesis of hydrogels as tissue-engineering scaffolds. Hydrogels are attractive scaffolding materials owing to their highly swollen network structure, ability to encapsulate cells and bioactive molecules, and efficient mass transfer. Various polymers, including natural, synthetic and natural/synthetic hybrid polymers, have been used to make hydrogels via chemical or physical crosslinking. Recently, bioactive synthetic hydrogels have emerged as promising scaffolds because they can provide molecularly tailored biofunctions and adjustable mechanical properties, as well as an extracellular matrix-like microenvironment for cell growth and tissue formation. This article addresses various strategies that have been explored to design synthetic hydrogels with extracellular matrix-mimetic bioactive properties, such as cell adhesion, proteolytic degradation and growth factor-binding.
Figures






References
-
- Lutolf MP. Biomaterials: Spotlight on hydrogels. Nat Mater. 2009;8(6):451–453. - PubMed
-
- Chung HK, Park TG. Self-assembled and nanostructured hydrogels for drug delivery and tissue engineering. Nano Today. 2009;4(5):429–437.
-
- Oh JK. Engineering of nanometer-sized cross-linked hydrogels for biomedical applications. Can J Chem. 2010;88(3):173–184.
-
- Ulijn RV, Bibi N, Jayawarna V, et al. Bioresponsive hydrogels. Mater Today. 2007;10(4):40–48.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
