Synaptic vesicle exocytosis
- PMID: 22026965
- PMCID: PMC3225952
- DOI: 10.1101/cshperspect.a005637
Synaptic vesicle exocytosis
Abstract
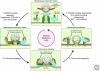
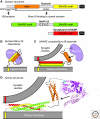
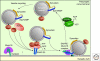
Presynaptic nerve terminals release neurotransmitters by synaptic vesicle exocytosis. Membrane fusion mediating synaptic exocytosis and other intracellular membrane traffic is affected by a universal machinery that includes SNARE (for "soluble NSF-attachment protein receptor") and SM (for "Sec1/Munc18-like") proteins. During fusion, vesicular and target SNARE proteins assemble into an α-helical trans-SNARE complex that forces the two membranes tightly together, and SM proteins likely wrap around assembling trans-SNARE complexes to catalyze membrane fusion. After fusion, SNARE complexes are dissociated by the ATPase NSF (for "N-ethylmaleimide sensitive factor"). Fusion-competent conformations of SNARE proteins are maintained by chaperone complexes composed of CSPα, Hsc70, and SGT, and by nonenzymatically acting synuclein chaperones; dysfunction of these chaperones results in neurodegeneration. The synaptic membrane-fusion machinery is controlled by synaptotagmin, and additionally regulated by a presynaptic protein matrix (the "active zone") that includes Munc13 and RIM proteins as central components.
Figures


References
-
- Augustin I, Rosenmund C, Südhof TC, Brose N 1999. Munc-13 is essential for fusion competence of glutamatergic synaptic vesicles. Nature 400: 457–461 - PubMed
-
- Blasi J, Chapman ER, Link E, Binz T, Yamasaki S, De Camilli P, Südhof TC, Niemann H, Jahn R 1993a. Botulinum neurotoxin A selectively cleaves the synaptic protein SNAP-25. Nature 365: 160–163 - PubMed
-
- Brose N, Hofmann K, Hata Y, Südhof TC 1995. Mammalian homologues of C. elegans unc-13 gene define novel family of C2-domain proteins. J Biol Chem 270: 25273–25280 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
