Novel vaginal microflora colonization model providing new insight into microbicide mechanism of action
- PMID: 22027006
- PMCID: PMC3202752
- DOI: 10.1128/mBio.00168-11
Novel vaginal microflora colonization model providing new insight into microbicide mechanism of action
Abstract
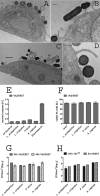
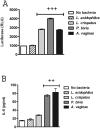
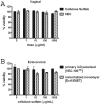
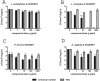
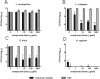
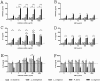
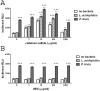
Several broad-spectrum microbicides, including cellulose sulfate (CS), have passed conventional preclinical and phase I clinical safety evaluation and yet have failed to protect women from acquiring HIV-1 in phase II/III trials. Concerns have been raised that current preclinical algorithms are deficient in addressing the complexity of the microflora-regulated vaginal mucosal barrier. We applied a novel microflora-colonized model to evaluate CS and hydroxyethylcellulose (HEC), which is used as a "universal placebo" in microbicide trials. Cervicovaginal epithelial cultures were colonized with normal vaginal microflora isolates representing common Lactobacillus species used as probiotics (L. acidophilus and L. crispatus) or Prevotella bivia and Atopobium vaginae, most prevalent in the disturbed microflora of bacterial vaginosis (BV). At baseline, all strains maintained constant epithelium-associated CFUs without inducing cytotoxicity and apoptosis. CS selectively reduced epithelium-associated CFUs and (to a lesser extent) planktonic CFUs, most significantly affecting L. crispatus. Inducing only minor changes in sterile epithelial cultures, CS induced expression of innate immunity mediators (RANTES, interleukin-8 [IL-8], and secretory leukocyte protease inhibitor [SLPI]) in microflora-colonized epithelia, most significantly potentiating effects of bacteria causing BV. In the absence of CS, all bacterial strains except L. acidophilus activated NF-κB, although IL-8 and RANTES levels were increased by the presence of BV-causing bacteria only. CS enhanced NF-κB activation in a dose-dependent manner under all conditions, including L. acidophilus colonization. HEC remained inert. These results offer insights into possible mechanisms of CS clinical failure. The bacterially colonized cervicovaginal model reveals unique aspects of microflora-epithelium-drug interactions and innate immunity in the female genital tract and should become an integral part of preclinical safety evaluation of anti-HIV microbicides and other vaginal formulations.
Importance: This report provides experimental evidence supporting the concept that the vaginal microflora regulates the epithelial innate immunity in a species- and strain-specific manner and that topically applied microbicides may alter both the bacterial and epithelial components of this homeostatic interaction. Our data also highlight the importance of differentiating the effects of biomedical interventions on epithelium-associated versus conventional planktonic bacterial growth when assessing vaginal mucosal health and immunity.
Figures
References
-
- Fichorova RN, Anderson DJ. 1999. Differential expression of immunobiological mediators by immortalized human cervical and vaginal epithelial cells. Biol. Reprod. 60:508–514 - PubMed
-
- Fichorova RN, Cronin AO, Lien E, Anderson DJ, Ingalls RR. 2002. Response to Neisseria gonorrhoeae by cervicovaginal epithelial cells occurs in the absence of Toll-like receptor 4-mediated signaling. J. Immunol. 168:2424–2432 - PubMed
-
- Wira CR, Fahey JV, Sentman CL, Pioli PA, Shen L. 2005. Innate and adaptive immunity in female genital tract: cellular responses and interactions. Immunol. Rev. 206:306–335 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

