The intracellular amino terminus plays a dominant role in desensitization of ATP-gated P2X receptor ion channels
- PMID: 22027824
- PMCID: PMC3247974
- DOI: 10.1074/jbc.M111.303917
The intracellular amino terminus plays a dominant role in desensitization of ATP-gated P2X receptor ion channels
Abstract
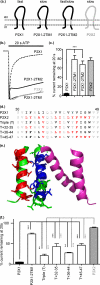
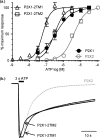
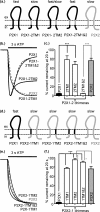
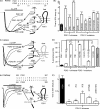
P2X receptors show marked variations in the time-course of response to ATP application from rapidly desensitizing P2X1 receptors to relatively sustained P2X2 receptors. In this study we have used chimeras between human P2X1 and P2X2 receptors in combination with mutagenesis to address the contribution of the extracellular ligand binding loop, the transmembrane channel, and the intracellular regions to receptor time-course. Swapping either the extracellular loop or both transmembrane domains (TM1 and -2) between the P2X1 and P2X2 receptors had no effect on the time-course of ATP currents in the recipient receptor. These results suggest that the agonist binding and channel-forming portions of the receptor do not play a major role in the control of the time-course. In contrast replacing the amino terminus of the P2X1 receptor with that from the non-desensitizing P2X2 receptor (P2X1-2N) slowed desensitization, and the mirror chimera induced rapid desensitization in the P2X2-1N chimera. These reciprocal effects on time-course can be replicated by changing four variant amino acids just before the first transmembrane (TM1) segment. These pre-TM1 residues also had a dominant effect on chimeras where both TMs had been transferred; mutating the variant amino acids 21-23 to those found in the P2X2 receptor removed desensitization from the P2X1-2TM1/-2 chimera, and the reciprocal mutants induced rapid desensitization in the non-desensitizing P2X2-1TM1/-2 chimera. These results suggest that the intracellular amino terminus, in particular the region just before TM1, plays a dominant role in the regulation of the time-course of ATP evoked P2X receptor currents.
Figures

References
-
- Burnstock G. (2006) Trends Pharmacol. Sci. 27, 166–176 - PubMed
-
- Surprenant A., North R. A. (2009) Annu. Rev. Physiol. 71, 333–359 - PubMed
-
- Oury C., Kuijpers M. J., Toth-Zsamboki E., Bonnefoy A., Danloy S., Vreys I., Feijge M. A., De Vos R., Vermylen J., Heemskerk J. W., Hoylaerts M. F. (2003) Blood 101, 3969–3976 - PubMed
-
- Mulryan K., Gitterman D. P., Lewis C. J., Vial C., Leckie B. J., Cobb A. L., Brown J. E., Conley E. C., Buell G., Pritchard C. A., Evans R. J. (2000) Nature 403, 86–89 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

