Galectin-9 protein expression in endothelial cells is positively regulated by histone deacetylase 3
- PMID: 22027828
- PMCID: PMC3243525
- DOI: 10.1074/jbc.M111.242289
Galectin-9 protein expression in endothelial cells is positively regulated by histone deacetylase 3
Abstract
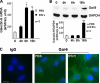
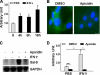
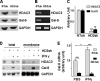
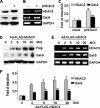
Galectin-9 expression in endothelial cells can be induced in response to inflammation. However, the mechanism of its expression remains unclear. In this study, we found that interferon-γ (IFN-γ) induced galectin-9 expression in human endothelial cells in a time-dependent manner, which coincided with the activation of histone deacetylase (HDAC). When endothelial cells were treated with the HDAC3 inhibitor, apicidin, or shRNA-HDAC3 knockdown, IFN-γ-induced galectin-9 expression was abolished. Overexpression of HDAC3 induced the interaction between phosphoinositol 3-kinase (PI3K) and IFN response factor 3 (IRF3), leading to IRF3 phosphorylation, nuclear translocation, and galectin-9 expression. HDAC3 functioned as a scaffold protein for PI3K/IRF3 interaction. In addition to galectin-9 expression, IFN-γ also induced galectin-9 location onto plasma membrane, which was HDAC3-independent. Importantly, HDAC3 was essential for the constitutive transcription of PI3K and IRF3, which might be responsible for the basal level of galectin-9 expression. The phosphorylation of IRF3 was essential for galectin-9 expression. This study provides new evidence that HDAC3 regulates galectin-9 expression in endothelial cells via interaction with PI3K-IRF3 signal pathway.
Figures


References
-
- Leffler H., Carlsson S., Hedlund M., Qian Y., Poirier F. (2004) Glycoconj. J. 19, 433–440 - PubMed
-
- Norling L. V., Perretti M., Cooper D. (2009) J. Endocrinol. 201, 169–184 - PubMed
-
- Yang R. Y., Rabinovich G. A., Liu F. T. (2008) Expert Rev. Mol. Med. 10, e17. - PubMed
-
- Asakura H., Kashio Y., Nakamura K., Seki M., Dai S., Shirato Y., Abedin M. J., Yoshida N., Nishi N., Imaizumi T., Saita N., Toyama Y., Takashima H., Nakamura T., Ohkawa M., Hirashima M. (2002) J. Immunol. 169, 5912–5918 - PubMed
-
- Matsumoto R., Matsumoto H., Seki M., Hata M., Asano Y., Kanegasaki S., Stevens R. L., Hirashima M. (1998) J. Biol. Chem. 273, 16976–16984 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

