Insulin resistance after a 72-h fast is associated with impaired AS160 phosphorylation and accumulation of lipid and glycogen in human skeletal muscle
- PMID: 22028408
- PMCID: PMC4971894
- DOI: 10.1152/ajpendo.00207.2011
Insulin resistance after a 72-h fast is associated with impaired AS160 phosphorylation and accumulation of lipid and glycogen in human skeletal muscle
Abstract
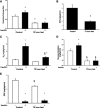
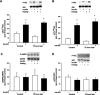
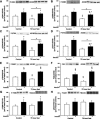
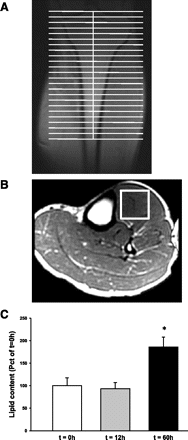
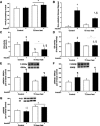
During fasting, human skeletal muscle depends on lipid oxidation for its energy substrate metabolism. This is associated with the development of insulin resistance and a subsequent reduction of insulin-stimulated glucose uptake. The underlying mechanisms controlling insulin action on skeletal muscle under these conditions are unresolved. In a randomized design, we investigated eight healthy subjects after a 72-h fast compared with a 10-h overnight fast. Insulin action on skeletal muscle was assessed by a hyperinsulinemic euglycemic clamp and by determining insulin signaling to glucose transport. In addition, substrate oxidation, skeletal muscle lipid content, regulation of glycogen synthesis, and AMPK signaling were assessed. Skeletal muscle insulin sensitivity was reduced profoundly in response to a 72-h fast and substrate oxidation shifted to predominantly lipid oxidation. This was associated with accumulation of both lipid and glycogen in skeletal muscle. Intracellular insulin signaling to glucose transport was impaired by regulation of phosphorylation at specific sites on AS160 but not TBC1D1, both key regulators of glucose uptake. In contrast, fasting did not impact phosphorylation of AMPK or insulin regulation of Akt, both of which are established upstream kinases of AS160. These findings show that insulin resistance in muscles from healthy individuals is associated with suppression of site-specific phosphorylation of AS160, without Akt or AMPK being affected. This impairment of AS160 phosphorylation, in combination with glycogen accumulation and increased intramuscular lipid content, may provide the underlying mechanisms for resistance to insulin in skeletal muscle after a prolonged fast.
Figures
References
-
- Bergman BC, Cornier MA, Horton TJ, Bessesen DH. Effects of fasting on insulin action and glucose kinetics in lean and obese men and women. Am J Physiol Endocrinol Metab 293: E1103–E1111, 2007. - PubMed
-
- Bernard C. Leçons Sur Les Propriétés Physiologiques et Les Altérations Pathologiques des Liquides de L'organisme. Paris: Ballière, 1859, p. 79.
-
- Bondar RJ, Mead DC. Evaluation of glucose-6-phosphate dehydrogenase from Leuconostoc mesenteroides in the hexokinase method for determining glucose in serum. Clin Chem 20: 586–590, 1974. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

