Testosterone regulates smooth muscle contractile pathways in the rat prostate: emphasis on PDE5 signaling
- PMID: 22028410
- PMCID: PMC3340899
- DOI: 10.1152/ajpendo.00458.2011
Testosterone regulates smooth muscle contractile pathways in the rat prostate: emphasis on PDE5 signaling
Abstract
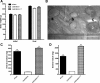
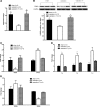
Testosterone (T) plays a permissive role in the development of benign prostatic hyperplasia (BPH), and phosphodiesterase 5 inhibitors (PDE5is) have been found to be effective for BPH and lower urinary tract symptoms (LUTS) in clinical trials. This study investigated the effect of T on smooth muscle (SM) contractile and regulatory signaling pathways, including PDE5 expression and functional activity in prostate in male rats (sham-operated, surgically castrated, and castrated with T supplementation). In vitro organ bath studies, real-time RT-PCR, Western blot analysis, and immunohistochemistry were performed. Castration heavily attenuated contractility, including sensitivity to phenylephrine with SM myosin immunostaining revealing a disrupted SM cell arrangement in the stroma. PDE5 was immunolocalized exclusively in the prostate stroma, and orchiectomy signficantly reduced PDE5 immunopositivity, mRNA, and protein expression, along with nNOS and ROKβ mRNA, whereas it increased eNOS plus α(1a) and α(1b) adrenoreceptor expression in castrated animals. The PDE5i zaprinast significantly increased prostate strip relaxation to the nitric oxide donor sodium nitroprusside (SNP) in control but not castrated rats. But SNP alone was more effective on castrated rats, comparable with sham treated with SNP plus zaprinast. T supplementation prevented or restored all above changes, including SNP and zaprinast in vitro responsiveness. In conclusion, our data show that T positively regulates PDE5 expression and functional activities in prostate, and T ablation not only suppresses prostate size but also reduces prostatic SM contractility, with several potential SM contraction/relaxation pathways implicated. Zaprinast findings strongly suggest a major role for PDE5/cGMP in this signaling cascade. PDE5 inhibition may represent a novel mechanism for treatment of BPH.
Figures



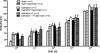
References
-
- Adelstein RS, Eisenberg E. Regulation and kinetics of the actin-myosin-ATP interaction. Annu Rev Biochem 49: 921–956, 1980 - PubMed
-
- Adolfsson PI, Ahlstrand C, Varenhorst E, Svensson SP. Lysophosphatidic acid stimulates proliferation of cultured smooth muscle cells from human BPH tissue: sildenafil and papaverin generate inhibition. Prostate 51: 50–58, 2002 - PubMed
-
- Andersson KE, de Groat WC, McVary KT, Lue TF, Maggi M, Roehrborn CG, Wyndaele JJ, Melby T, Viktrup L. Tadalafil for the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia: pathophysiology and mechanism(s) of action. Neurourol Urodyn 30: 292–301, 2011 - PubMed
-
- Andric SA, Janjic MM, Stojkov NJ, Kostic TS. Sildenafil treatment in vivo stimulates Leydig cell steroidogenesis via the cAMP/cGMP signaling pathway. Am J Physiol Endocrinol Metab 299: E544–E550, 2010 - PubMed
-
- Antonioli E, Cardoso AB, Carvalho HF. Effects of long-term castration on the smooth muscle cell phenotype of the rat ventral prostate. J Androl 28: 777–783, 2007 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

