Exhausted cytotoxic control of Epstein-Barr virus in human lupus
- PMID: 22028659
- PMCID: PMC3197610
- DOI: 10.1371/journal.ppat.1002328
Exhausted cytotoxic control of Epstein-Barr virus in human lupus
Abstract
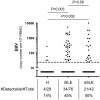
Systemic Lupus Erythematosus (SLE) pathology has long been associated with an increased Epstein-Barr Virus (EBV) seropositivity, viremia and cross-reactive serum antibodies specific for both virus and self. It has therefore been postulated that EBV triggers SLE immunopathology, although the mechanism remains elusive. Here, we investigate whether frequent peaks of EBV viral load in SLE patients are a consequence of dysfunctional anti-EBV CD8+ T cell responses. Both inactive and active SLE patients (n = 76 and 42, respectively), have significantly elevated EBV viral loads (P = 0.003 and 0.002, respectively) compared to age- and sex-matched healthy controls (n = 29). Interestingly, less EBV-specific CD8+ T cells are able to secrete multiple cytokines (IFN-γ, TNF-α, IL-2 and MIP-1β) in inactive and active SLE patients compared to controls (P = 0.0003 and 0.0084, respectively). Moreover, EBV-specific CD8+ T cells are also less cytotoxic in SLE patients than in controls (CD107a expression: P = 0.0009, Granzyme B release: P = 0.0001). Importantly, cytomegalovirus (CMV)-specific responses were not found significantly altered in SLE patients. Furthermore, we demonstrate that EBV-specific CD8+ T cell impairment is a consequence of their Programmed Death 1 (PD-1) receptor up-regulation, as blocking this pathway reverses the dysfunctional phenotype. Finally, prospective monitoring of lupus patients revealed that disease flares precede EBV reactivation. In conclusion, EBV-specific CD8+ T cell responses in SLE patients are functionally impaired, but EBV reactivation appears to be an aggravating consequence rather than a cause of SLE immunopathology. We therefore propose that autoimmune B cell activation during flares drives frequent EBV reactivation, which contributes in a vicious circle to the perpetuation of immune activation in SLE patients.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- James JA, Harley JB, Scofield RH. Role of viruses in systemic lupus erythematosus and Sjogren syndrome. Curr Opin Rheumatol. 2001;13:370–376. - PubMed
-
- Evans AS, Rothfield NF, Niederman JC. Raised antibody titres to E.B. virus in systemic lupus erythematosus. Lancet. 1971;1:167–168. - PubMed
-
- Barzilai O, Ram M, Shoenfeld Y. Viral infection can induce the production of autoantibodies. Curr Opin Rheumatol. 2007;19:636–643. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

