Fitting the elementary rate constants of the P-gp transporter network in the hMDR1-MDCK confluent cell monolayer using a particle swarm algorithm
- PMID: 22028772
- PMCID: PMC3196501
- DOI: 10.1371/journal.pone.0025086
Fitting the elementary rate constants of the P-gp transporter network in the hMDR1-MDCK confluent cell monolayer using a particle swarm algorithm
Abstract
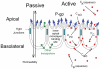
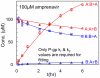
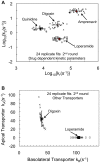
P-glycoprotein, a human multidrug resistance transporter, has been extensively studied due to its importance to human health and disease. In order to understand transport kinetics via P-gp, confluent cell monolayers overexpressing P-gp are widely used. The purpose of this study is to obtain the mass action elementary rate constants for P-gp's transport and to functionally characterize members of P-gp's network, i.e., other transporters that transport P-gp substrates in hMDR1-MDCKII confluent cell monolayers and are essential to the net substrate flux. Transport of a range of concentrations of amprenavir, loperamide, quinidine and digoxin across the confluent monolayer of cells was measured in both directions, apical to basolateral and basolateral to apical. We developed a global optimization algorithm using the Particle Swarm method that can simultaneously fit all datasets to yield accurate and exhaustive fits of these elementary rate constants. The statistical sensitivity of the fitted values was determined by using 24 identical replicate fits, yielding simple averages and standard deviations for all of the kinetic parameters, including the efflux active P-gp surface density. Digoxin required additional basolateral and apical transporters, while loperamide required just a basolateral tranporter. The data were better fit by assuming bidirectional transporters, rather than active importers, suggesting that they are not MRP or active OATP transporters. The P-gp efflux rate constants for quinidine and digoxin were about 3-fold smaller than reported ATP hydrolysis rate constants from P-gp proteoliposomes. This suggests a roughly 3∶1 stoichiometry between ATP hydrolysis and P-gp transport for these two drugs. The fitted values of the elementary rate constants for these P-gp substrates support the hypotheses that the selective pressures on P-gp are to maintain a broad substrate range and to keep xenobiotics out of the cytosol, but not out of the apical membrane.
Conflict of interest statement
Figures


References
-
- Endres CJ, Hsiao P, Chung FS, Unadkat JD. The role of transporters in drug interactions. Eur J Pharm Sci. 2006;27:501–517. - PubMed
-
- Shitara Y, Horiea T, Sugiyama Y. Transporters as a determinant of drug clearance and tissue distribution. Eur J Pharm Sci. 2006;27:425–446. - PubMed
-
- Sharom FJ. Drug Transporters: Molecular characterization and role in drug disposition. Hoboken, NJ: John Wiley & Sons, Inc; 2007.
-
- Higgins CF, Linton K. The ATP switch model for ATP transporters. Nature Struct & Mol Biol. 2004;11:918–926. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

