Salivary PYY: a putative bypass to satiety
- PMID: 22028819
- PMCID: PMC3189958
- DOI: 10.1371/journal.pone.0026137
Salivary PYY: a putative bypass to satiety
Abstract
Peptide YY(3-36) is a satiation hormone released postprandially into the bloodstream from L-endocrine cells in the gut epithelia. In the current report, we demonstrate PYY(3-36) is also present in murine as well as in human saliva. In mice, salivary PYY(3-36) derives from plasma and is also synthesized in the taste cells in taste buds of the tongue. Moreover, the cognate receptor Y2R is abundantly expressed in the basal layer of the progenitor cells of the tongue epithelia and von Ebner's gland. The acute augmentation of salivary PYY(3-36) induced stronger satiation as demonstrated in feeding behavioral studies. The effect is mediated through the activation of the specific Y2 receptor expressed in the lingual epithelial cells. In a long-term study involving diet-induced obese (DIO) mice, a sustained increase in PYY(3-36) was achieved using viral vector-mediated gene delivery targeting salivary glands. The chronic increase in salivary PYY(3-36) resulted in a significant long-term reduction in food intake (FI) and body weight (BW). Thus this study provides evidence for new functions of the previously characterized gut peptide PYY(3-36) suggesting a potential simple and efficient alternative therapeutic approach for the treatment of obesity.
Conflict of interest statement
Figures

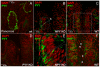
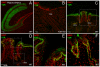
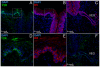
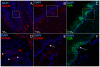

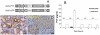

References
-
- Vallejo G, Mead PM, Gaynor DH, Devlin JT, Robbins DC. Characterization of immunoreactive insulin in human saliva: evidence against production in situ. Diabetologia. 1984;27:437–440. - PubMed
-
- Groschl M, Rauh M, Wagner R, Neuhuber W, Metzler M, et al. Identification of leptin in human saliva. J Clin Endocrinol Metab. 2001;86:5234–5239. - PubMed
-
- Toda M, Tsukinoki R, Morimoto K. Measurement of salivary adiponectin levels. Acta Diabetol. 2007;44:20–22. - PubMed
-
- Herness MS. Vasoactive intestinal peptide-like immunoreactivity in rodent taste cells. Neuroscience. 1989;33:411–419. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

