Evidence for oxidative stress and defective antioxidant response in guinea pigs with tuberculosis
- PMID: 22028843
- PMCID: PMC3196542
- DOI: 10.1371/journal.pone.0026254
Evidence for oxidative stress and defective antioxidant response in guinea pigs with tuberculosis
Abstract
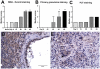
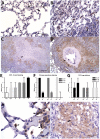
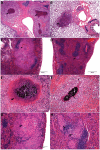
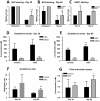
The development of granulomatous inflammation with caseous necrosis is an important but poorly understood manifestation of tuberculosis in humans and some animal models. In this study we measured the byproducts of oxidative stress in granulomatous lesions as well as the systemic antioxidant capacity of BCG vaccinated and non-vaccinated guinea pigs experimentally infected with Mycobacterium tuberculosis. In non-vaccinated guinea pigs, oxidative stress was evident within 2 weeks of infection as measured by a decrease in the serum total antioxidant capacity and blood glutathione levels accompanied by an increase in malondialdehyde, a byproduct of lipid peroxidation, within lesions. Despite a decrease in total and reduced blood glutathione concentrations, there was an increase in lesion glutathione by immunohistochemistry in response to localized oxidative stress. In addition there was an increase in the expression of the host transcription factor nuclear erythroid 2 p45-related factor 2 (Nrf2), which regulates several protein and non-proteins antioxidants, including glutathione. Despite the increase in cytoplasmic expression of Nrf2, immunohistochemical staining revealed a defect in Nrf2 nuclear translocation within granulomatous lesions as well as a decrease in the expression of the Nrf2-regulated antioxidant protein NQO1. Treating M. tuberculosis-infected guinea pigs with the antioxidant drug N-acetyl cysteine (NAC) partially restored blood glutathione concentrations and the serum total antioxidant capacity. Treatment with NAC also decreased spleen bacterial counts, as well as decreased the lung and spleen lesion burden and the severity of lesion necrosis. These data suggest that the progressive oxidative stress during experimental tuberculosis in guinea pigs is due in part to a defect in host antioxidant defenses, which, we show here, can be partially restored with antioxidant treatment. These data suggest that the therapeutic strategies that reduce oxidant-mediated tissue damage may be beneficial as an adjunct therapy in the treatment and prevention of tuberculosis in humans.
Conflict of interest statement
Figures

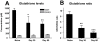



References
-
- Frieden TR, Sterling TR, Munsiff SS, Watt CJ, Dye C. Tuberculosis. The Lancet. 2003;362:887–899. - PubMed
-
- WHO. 2010. Global TB control report 2010.
-
- Espinal MA, Raviglione MC. Global Epidemiology of Tuberculosis. In: M MM, editor. Tuberculosis. Berlin: Springer-Verlag; 2003. pp. 33–43.
-
- Canetti G. The Tubercle Bacillus in the Pulmonary Lesion of Man; Histobacteriology and its bearing on the therapy of pulmonary tuberculosis. New York: Springer Publishing Company, Inc; 1955.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

