Attenuated inflammatory response in aged mice brains following stroke
- PMID: 22028848
- PMCID: PMC3196544
- DOI: 10.1371/journal.pone.0026288
Attenuated inflammatory response in aged mice brains following stroke
Abstract
Background: Increased age is a major risk factor for stroke incidence, post-ischemic mortality, and severe and long-term disability. Stroke outcome is considerably influenced by post-ischemic mechanisms. We hypothesized that the inflammatory response following an ischemic injury is altered in aged organisms.
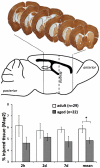
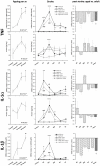
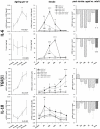
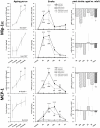
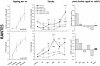
Methods and results: To that end, we analyzed the expression pattern of pro-inflammatory cytokines (TNF, IL-1α, IL-1β, IL-6), anti-inflammatory cytokines (IL-10, TGFβ1), and chemokines (Mip-1α, MCP-1, RANTES) of adult (2 months) and aged (24 months) mice brains at different reperfusion times (6 h, 12 h, 24 h, 2 d, 7 d) following transient occlusion of the middle cerebral artery. The infarct size was assessed to monitor possible consequences of an altered inflammatory response in aged mice. Our data revealed an increased neuro-inflammation with age. Above all, we found profound age-related alterations in the reaction to stroke. The response of pro-inflammatory cytokines (TNF, and IL-1β) and the level of chemokines (Mip-1α, and MCP-1) were strongly diminished in the aged post-ischemic brain tissue. IL-6 showed the strongest age-dependent decrease in its post-ischemic expression profile. Anti-inflammatory cytokines (TGFβ1, and IL-10) revealed no significant age dependency after ischemia. Aged mice brains tend to develop smaller infarcts.
Conclusion: The attenuated inflammatory response to stroke in aged animals may contribute to their smaller infarcts. The results presented here highlight the importance of using aged animals to investigate age-associated diseases like stroke, and should be considered as a major prerequisite in the development of age-adjusted therapeutic interventions.
Conflict of interest statement
Figures

References
-
- Truelsen T, Piechowski-Jozwiak B, Bonita R, Mathers C, Bogousslavsky J, et al. Stroke incidence and prevalence in Europe: a review of available data. Eur J Neurol. 2006;13:581–598. - PubMed
-
- Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet. 2008;371:1612–1623. - PubMed
-
- Saposnik G, Black SE, Hakim A, Fang J, Tu JV, et al. Age disparities in stroke quality of care and delivery of health services. Stroke. 2009;40:3328–3335. - PubMed
-
- O'Collins VE, Macleod MR, Donnan GA, Horky LL, van der Worp BH, et al. 1,026 experimental treatments in acute stroke. Ann Neurol. 2006;59:467–477. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

