Development and inter-laboratory validation of unlabeled probe melting curve analysis for detection of JAK2 V617F mutation in polycythemia vera
- PMID: 22028900
- PMCID: PMC3197667
- DOI: 10.1371/journal.pone.0026534
Development and inter-laboratory validation of unlabeled probe melting curve analysis for detection of JAK2 V617F mutation in polycythemia vera
Abstract
Background: JAK2 V617F, a somatic point mutation that leads to constitutive JAK2 phosphorylation and kinase activation, has been incorporated into the WHO classification and diagnostic criteria of myeloid neoplasms. Although various approaches such as restriction fragment length polymorphism, amplification refractory mutation system and real-time PCR have been developed for its detection, a generic rapid closed-tube method, which can be utilized on routine genetic testing instruments with stability and cost-efficiency, has not been described.
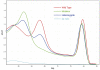
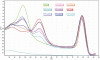
Methodology/principal findings: Asymmetric PCR for detection of JAK2 V617F with a 3'-blocked unlabeled probe, saturate dye and subsequent melting curve analysis was performed on a Rotor-Gene® Q real-time cycler to establish the methodology. We compared this method to the existing amplification refractory mutation systems and direct sequencing. Hereafter, the broad applicability of this unlabeled probe melting method was also validated on three diverse real-time systems (Roche LightCycler® 480, Applied Biosystems ABI® 7500 and Eppendorf Mastercycler® ep realplex) in two different laboratories. The unlabeled probe melting analysis could genotype JAK2 V617F mutation explicitly with a 3% mutation load detecting sensitivity. At level of 5% mutation load, the intra- and inter-assay CVs of probe-DNA heteroduplex (mutation/wild type) covered 3.14%/3.55% and 1.72%/1.29% respectively. The method could equally discriminate mutant from wild type samples on the other three real-time instruments.
Conclusions: With a high detecting sensitivity, unlabeled probe melting curve analysis is more applicable to disclose JAK2 V617F mutation than conventional methodologies. Verified with the favorable inter- and intra-assay reproducibility, unlabeled probe melting analysis provided a generic mutation detecting alternative for real-time instruments.
Conflict of interest statement
Figures

Similar articles
-
Melting point assay for the JAK2 V617F mutation, comparison with amplification refractory mutation system (ARMS) in diagnostic samples, and implications for daily routine.Mol Diagn Ther. 2010 Jun 1;14(3):185-90. doi: 10.1007/BF03256372. Mol Diagn Ther. 2010. PMID: 20560681
-
A sensitive and reliable semi-quantitative real-time PCR assay to detect JAK2 V617F in blood.Hematol Oncol. 2006 Dec;24(4):227-33. doi: 10.1002/hon.800. Hematol Oncol. 2006. PMID: 17006961
-
Toward point-of-care testing for JAK2 V617F mutation on a microchip.J Chromatogr A. 2015 Sep 4;1410:28-34. doi: 10.1016/j.chroma.2015.07.079. Epub 2015 Jul 23. J Chromatogr A. 2015. PMID: 26235214
-
JAK2 V617F/C618R mutation in a patient with polycythemia vera: a case study and review of the literature.Cancer Genet Cytogenet. 2009 Feb;189(1):43-7. doi: 10.1016/j.cancergencyto.2008.09.010. Cancer Genet Cytogenet. 2009. PMID: 19167611 Review.
-
A refined diagnostic algorithm for polycythemia vera that incorporates mutation screening for JAK2(V617F).Curr Hematol Malig Rep. 2006 Jun;1(2):81-6. doi: 10.1007/s11899-006-0027-2. Curr Hematol Malig Rep. 2006. PMID: 20425336 Review.
Cited by
-
A multiplex snapback primer system for the enrichment and detection of JAK2 V617F and MPL W515L/K mutations in Philadelphia-negative myeloproliferative neoplasms.Biomed Res Int. 2014;2014:458457. doi: 10.1155/2014/458457. Epub 2014 Mar 5. Biomed Res Int. 2014. PMID: 24729973 Free PMC article.
-
Development of a cost-effective method for capripoxvirus genotyping using snapback primer and dsDNA intercalating dye.PLoS One. 2013 Oct 7;8(10):e75971. doi: 10.1371/journal.pone.0075971. eCollection 2013. PLoS One. 2013. PMID: 24116084 Free PMC article.
-
Elucidation of sequence polymorphism in fuzzless-seed cotton lines.Mol Genet Genomics. 2021 Jan;296(1):193-206. doi: 10.1007/s00438-020-01736-z. Epub 2020 Nov 3. Mol Genet Genomics. 2021. PMID: 33141290
-
Detection of the G17V RHOA mutation in angioimmunoblastic T-cell lymphoma and related lymphomas using quantitative allele-specific PCR.PLoS One. 2014 Oct 13;9(10):e109714. doi: 10.1371/journal.pone.0109714. eCollection 2014. PLoS One. 2014. PMID: 25310466 Free PMC article.
-
The mutation profile of JAK2 and CALR in Chinese Han patients with Philadelphia chromosome-negative myeloproliferative neoplasms.J Hematol Oncol. 2014 Jul 15;7:48. doi: 10.1186/s13045-014-0048-6. J Hematol Oncol. 2014. PMID: 25023898 Free PMC article.
References
-
- Levine RL, Wadleigh M, Cools J, Ebert BL, Wernig G, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7:387–397. - PubMed
-
- Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. The Lancet. 2005;365:1054–1061. - PubMed
-
- Jones AV, Kreil S, Zoi K, Waghorn K, Curtis C, et al. Widespread occurrence of the JAK2 V617F mutation in chronic myeloproliferative disorders. Blood. 2005;106:2162. - PubMed
-
- Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. New Engl J Med. 2005;352:1779–1790. - PubMed
-
- Swerdlow S, Campo E, Harris NL, Jaffe ES, Pileri SA, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. WHO Classification of Tumours. 4th ed. Lyon, France: IARC; 2008.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

