Paranodal myelin retraction in relapsing experimental autoimmune encephalomyelitis visualized by coherent anti-Stokes Raman scattering microscopy
- PMID: 22029353
- PMCID: PMC3206924
- DOI: 10.1117/1.3638180
Paranodal myelin retraction in relapsing experimental autoimmune encephalomyelitis visualized by coherent anti-Stokes Raman scattering microscopy
Abstract
How demyelination is initiated is a standing question for pathology of multiple sclerosis. By label-free coherent anti-Stokes Raman scattering (CARS) imaging of myelin lipids, we investigate myelin integrity in the lumbar spinal cord tissue isolated from naïve SJL mice, and from mice at the onset, peak acute, and remission stages of relapsing experimental autoimmune encephalomyelitis (EAE). Progressive demyelinating disease is initially characterized by the retraction of paranodal myelin both at the onset of disease and at the borders of acute demyelinating lesions. Myelin retraction is confirmed by elongated distribution of neurofascin proteins visualized by immunofluorescence. The disruption of paranodal myelin subsequently exposes Kv1.2 channels at the juxtaparanodes and lead to the displacement of Kv1.2 channels to the paranodal and nodal domains. Paranodal myelin is partially restored during disease remission, indicating spontaneous myelin regeneration. These findings suggest that paranodal domain injury precedes formation of internodal demyelinating lesions in relapsing EAE. Our results also demonstrate that CARS microscopy is an effective readout of myelin disease burden.
Figures




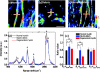



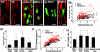
References
-
- Tait S., Gunn-Moore F., Collinson J. M., Huang J., Lubetzki C., Pedraza L., Sherman D. L., Colman D. R., and Brophy P. J., “An oligodendrocyte cell adhesion molecule at the site of assembly of the paranodal axo-glial junction,” J. Cell Biol. 150, 657–666 (2000).10.1083/jcb.150.3.657 - DOI - PMC - PubMed
-
- Charles P., Tait S., Faivre-Sarrailh C., Barbin G., Gunn-Moore F., Denisenko-Nehrbass N., Guennoc A., Girault J., Brophy P. J., and Lubetzki C., “Neurofascin is a glial receptor for the paranodin/Caspr-contactin axonal complex at the axoglial junction,” Curr. Biol. 12, 217–220 (2002).10.1016/S0960-9822(01)00680-7 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

