Visceral hyperalgesia induced by forebrain-specific suppression of native Kv7/KCNQ/M-current in mice
- PMID: 22029713
- PMCID: PMC3214183
- DOI: 10.1186/1744-8069-7-84
Visceral hyperalgesia induced by forebrain-specific suppression of native Kv7/KCNQ/M-current in mice
Abstract
Background: Dysfunction of brain-gut interaction is thought to underlie visceral hypersensitivity which causes unexplained abdominal pain syndromes. However, the mechanism by which alteration of brain function in the brain-gut axis influences the perception of visceral pain remains largely elusive. In this study we investigated whether altered brain activity can generate visceral hyperalgesia.
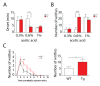
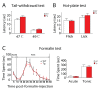
Results: Using a forebrain specific αCaMKII promoter, we established a line of transgenic (Tg) mice expressing a dominant-negative pore mutant of the Kv7.2/KCNQ2 channel which suppresses native KCNQ/M-current and enhances forebrain neuronal excitability. Brain slice recording of hippocampal pyramidal neurons from these Tg mice confirmed the presence of hyperexcitable properties with increased firing. Behavioral evaluation of Tg mice exhibited increased sensitivity to visceral pain induced by intraperitoneal (i.p.) injection of either acetic acid or magnesium sulfate, and intracolon capsaicin stimulation, but not cutaneous sensation for thermal or inflammatory pain. Immunohistological staining showed increased c-Fos expression in the somatosensory SII cortex and insular cortex of Tg mice that were injected intraperitoneally with acetic acid. To mimic the effect of cortical hyperexcitability on visceral hyperalgesia, we injected KCNQ/M channel blocker XE991 into the lateral ventricle of wild type (WT) mice. Intracerebroventricular injection of XE991 resulted in increased writhes of WT mice induced by acetic acid, and this effect was reversed by co-injection of the channel opener retigabine.
Conclusions: Our findings provide evidence that forebrain hyperexcitability confers visceral hyperalgesia, and suppression of central hyperexcitability by activation of KCNQ/M-channel function may provide a therapeutic potential for treatment of abdominal pain syndromes.
Figures


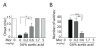


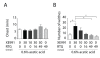
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

