Pharmacokinetics of prolonged infusion of high-dose dexmedetomidine in critically ill patients
- PMID: 22030215
- PMCID: PMC3334808
- DOI: 10.1186/cc10518
Pharmacokinetics of prolonged infusion of high-dose dexmedetomidine in critically ill patients
Abstract
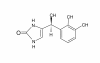
Introduction: Only limited information exists on the pharmacokinetics of prolonged (> 24 hours) and high-dose dexmedetomidine infusions in critically ill patients. The aim of this study was to characterize the pharmacokinetics of long dexmedetomidine infusions and to assess the dose linearity of high doses. Additionally, we wanted to quantify for the first time in humans the concentrations of H-3, a practically inactive metabolite of dexmedetomidine.
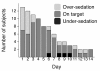
Methods: Thirteen intensive care patients with mean age of 57 years and Simplified Acute Physiology Score (SAPS) II score of 45 were included in the study. Dexmedetomidine infusion was commenced by using a constant infusion rate for the first 12 hours. After the first 12 hours, the infusion rate of dexmedetomidine was titrated between 0.1 and 2.5 μg/kg/h by using predefined dose levels to maintain sedation in the range of 0 to -3 on the Richmond Agitation-Sedation Scale. Dexmedetomidine was continued as long as required to a maximum of 14 days. Plasma dexmedetomidine and H-3 metabolite concentrations were measured, and pharmacokinetic variables were calculated with standard noncompartmental methods. Safety and tolerability were assessed by adverse events, cardiovascular signs, and laboratory tests.
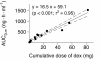
Results: The following geometric mean values (coefficient of variation) were calculated: length of infusion, 92 hours (117%); dexmedetomidine clearance, 39.7 L/h (41%); elimination half-life, 3.7 hours (38%); and volume of distribution during the elimination phase, 223 L (35%). Altogether, 116 steady-state concentrations were found in 12 subjects. The geometric mean value for clearance at steady state was 53.1 L/h (55%). A statistically significant linear relation (r2 = 0.95; P < 0.001) was found between the areas under the dexmedetomidine plasma concentration-time curves and cumulative doses of dexmedetomidine. The elimination half-life of H-3 was 9.1 hours (37%). The ratio of AUC0-∞ of H-3 metabolite to that of dexmedetomidine was 1.47 (105%), ranging from 0.29 to 4.4. The ratio was not statistically significantly related to the total dose of dexmedetomidine or the duration of the infusion.
Conclusions: The results suggest linear pharmacokinetics of dexmedetomidine up to the dose of 2.5 μg/kg/h. Despite the high dose and prolonged infusions, safety findings were as expected for dexmedetomidine and the patient population.
Trial registration: ClinicalTrials.gov: NCT00747721.
Figures


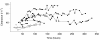
Similar articles
-
Dexmedetomidine Pharmacology in Neonates and Infants After Open Heart Surgery.Anesth Analg. 2016 May;122(5):1556-66. doi: 10.1213/ANE.0000000000000869. Anesth Analg. 2016. PMID: 26218862 Clinical Trial.
-
Population pharmacokinetics of dexmedetomidine during long-term sedation in intensive care patients.Br J Anaesth. 2012 Mar;108(3):460-8. doi: 10.1093/bja/aer441. Epub 2012 Jan 25. Br J Anaesth. 2012. PMID: 22277665 Clinical Trial.
-
Altered Pharmacokinetics in Prolonged Infusions of Sedatives and Analgesics Among Adult Critically Ill Patients: A Systematic Review.Clin Ther. 2018 Sep;40(9):1598-1615.e2. doi: 10.1016/j.clinthera.2018.07.021. Epub 2018 Aug 31. Clin Ther. 2018. PMID: 30173953
-
A phase II/III, multicenter, safety, efficacy, and pharmacokinetic study of dexmedetomidine in preterm and term neonates.J Pediatr. 2014 Feb;164(2):276-82.e1-3. doi: 10.1016/j.jpeds.2013.10.002. Epub 2013 Nov 14. J Pediatr. 2014. PMID: 24238862 Clinical Trial.
-
Prolonged infusions of dexmedetomidine in critically ill patients.Am J Health Syst Pharm. 2010 Aug;67(15):1246-53. doi: 10.2146/ajhp090300. Am J Health Syst Pharm. 2010. PMID: 20651314 Review.
Cited by
-
Population pharmacokinetics of dexmedetomidine in critically ill patients.Clin Drug Investig. 2013 Aug;33(8):579-87. doi: 10.1007/s40261-013-0101-1. Clin Drug Investig. 2013. PMID: 23839483 Free PMC article. Clinical Trial.
-
Dexmedetomidine-Associated Hyperpyrexia in Three Critically Ill Patients With Coronavirus Disease 2019.Crit Care Explor. 2020 Sep 15;2(9):e0213. doi: 10.1097/CCE.0000000000000213. eCollection 2020 Sep. Crit Care Explor. 2020. PMID: 32984835 Free PMC article.
-
Variant-based heritability assessment of dexmedetomidine and fentanyl clearance in pediatric patients.Clin Transl Sci. 2023 Sep;16(9):1628-1638. doi: 10.1111/cts.13574. Epub 2023 Jun 26. Clin Transl Sci. 2023. PMID: 37353859 Free PMC article.
-
Sedation in intensive care unit: Is Dexmedetomidine the best choice?Int J Crit Illn Inj Sci. 2012 Jan;2(1):3-5. doi: 10.4103/2229-5151.94866. Int J Crit Illn Inj Sci. 2012. PMID: 22624094 Free PMC article. No abstract available.
-
Comparison of Intravenous Versus Nebulized Dexmedetomidine for Laryngoscopy and Intubation-Induced Sympathoadrenal Stress Response Attenuation.Anesth Pain Med. 2022 Nov 23;12(5):e132607. doi: 10.5812/aapm-132607. eCollection 2022 Oct. Anesth Pain Med. 2022. PMID: 36937178 Free PMC article.
References
-
- Devlin JW, Mallow-Corbett S, Riker RR. Adverse drug events associated with the use of analgesics, sedatives, and antipsychotics in the intensive care unit. Crit Care Med. 2010;38:S231–S243. - PubMed
-
- Karol MD, Maze M. Pharmacokinetics and interaction pharmacodynamics of dexmedetomidine in humans. Baillières Clin Anaesthesiol. 2000;14:261–269.
-
- Orion Corporation. Dexdor® [summary of product characteristics] Espoo, Finland: Orion Corporation; 2011.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

