Central nervous system pericytes in health and disease
- PMID: 22030551
- PMCID: PMC4020628
- DOI: 10.1038/nn.2946
Central nervous system pericytes in health and disease
Abstract
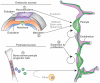
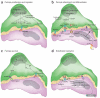
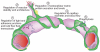
Pericytes are uniquely positioned within the neurovascular unit to serve as vital integrators, coordinators and effectors of many neurovascular functions, including angiogenesis, blood-brain barrier (BBB) formation and maintenance, vascular stability and angioarchitecture, regulation of capillary blood flow and clearance of toxic cellular byproducts necessary for proper CNS homeostasis and neuronal function. New studies have revealed that pericyte deficiency in the CNS leads to BBB breakdown and brain hypoperfusion resulting in secondary neurodegenerative changes. Here we review recent progress in understanding the biology of CNS pericytes and their role in health and disease.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

