Bifunctional RNAs targeting the intronic splicing silencer N1 increase SMN levels and reduce disease severity in an animal model of spinal muscular atrophy
- PMID: 22031236
- PMCID: PMC3255575
- DOI: 10.1038/mt.2011.232
Bifunctional RNAs targeting the intronic splicing silencer N1 increase SMN levels and reduce disease severity in an animal model of spinal muscular atrophy
Abstract
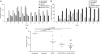
Spinal muscular atrophy (SMA) is a neurodegenerative disease caused by loss of survival motor neuron-1 (SMN1). A nearly identical copy gene, SMN2, is present in all SMA patients. Although the SMN2 coding sequence has the potential to produce full-length SMN, nearly 90% of SMN2-derived transcripts are alternatively spliced and encode a truncated protein. SMN2, however, is an excellent therapeutic target. Previously, we developed antisense-based oligonucleotides (bifunctional RNAs) that specifically recruit SR/SR-like splicing factors and target a negative regulator of SMN2 exon-7 inclusion within intron-6. As a means to optimize the antisense sequence of the bifunctional RNAs, we chose to target a potent intronic repressor downstream of SMN2 exon 7, called intronic splicing silencer N1 (ISS-N1). We developed RNAs that specifically target ISS-N1 and concurrently recruit the modular SR proteins SF2/ASF or hTra2β1. RNAs were directly injected in the brains of SMA mice. Bifunctional RNA injections were able to elicit robust induction of SMN protein in the brain and spinal column of neonatal SMA mice. Importantly, hTra2β1-ISS-N1 and SF2/ASF-ISS-N1 bifunctional RNAs significantly extended lifespan and increased weight in the SMNΔ7 mice. This technology has direct implications for SMA therapy and provides similar therapeutic strategies for other diseases caused by aberrant splicing.
Figures


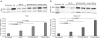


References
-
- Crawford TO., and, Pardo CA. The neurobiology of childhood spinal muscular atrophy. Neurobiol Dis. 1996;3:97–110. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

