Reprogramming adult dermis to a neonatal state through epidermal activation of β-catenin
- PMID: 22031549
- PMCID: PMC3210498
- DOI: 10.1242/dev.064592
Reprogramming adult dermis to a neonatal state through epidermal activation of β-catenin
Abstract
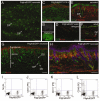
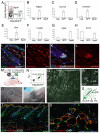
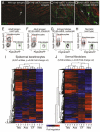
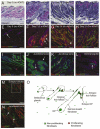
Hair follicle formation depends on reciprocal epidermal-dermal interactions and occurs during skin development, but not in adult life. This suggests that the properties of dermal fibroblasts change during postnatal development. To examine this, we used a PdgfraEGFP mouse line to isolate GFP-positive fibroblasts from neonatal skin, adult telogen and anagen skin and adult skin in which ectopic hair follicles had been induced by transgenic epidermal activation of β-catenin (EF skin). We also isolated epidermal cells from each mouse. The gene expression profile of EF epidermis was most similar to that of anagen epidermis, consistent with activation of β-catenin signalling. By contrast, adult dermis with ectopic hair follicles more closely resembled neonatal dermis than adult telogen or anagen dermis. In particular, genes associated with mitosis were upregulated and extracellular matrix-associated genes were downregulated in neonatal and EF fibroblasts. We confirmed that sustained epidermal β-catenin activation stimulated fibroblasts to proliferate to reach the high cell density of neonatal skin. In addition, the extracellular matrix was comprehensively remodelled, with mature collagen being replaced by collagen subtypes normally present only in developing skin. The changes in proliferation and extracellular matrix composition originated from a specific subpopulation of fibroblasts located beneath the sebaceous gland. Our results show that adult dermis is an unexpectedly plastic tissue that can be reprogrammed to acquire the molecular, cellular and structural characteristics of neonatal dermis in response to cues from the overlying epidermis.
Figures



References
-
- Chan E. F., Gat U., McNiff J. M., Fuchs E. (1999). A common human skin tumour is caused by activating mutations in β-catenin. Nat. Genet. 21, 410–413 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

