Psb27, a transiently associated protein, binds to the chlorophyll binding protein CP43 in photosystem II assembly intermediates
- PMID: 22031695
- PMCID: PMC3215006
- DOI: 10.1073/pnas.1111597108
Psb27, a transiently associated protein, binds to the chlorophyll binding protein CP43 in photosystem II assembly intermediates
Abstract
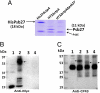
Photosystem II (PSII), a large multisubunit pigment-protein complex localized in the thylakoid membrane of cyanobacteria and chloroplasts, mediates light-driven evolution of oxygen from water. Recently, a high-resolution X-ray structure of the mature PSII complex has become available. Two PSII polypeptides, D1 and CP43, provide many of the ligands to an inorganic Mn(4)Ca center that is essential for water oxidation. Because of its unusual redox chemistry, PSII often undergoes degradation followed by stepwise assembly. Psb27, a small luminal polypeptide, functions as an important accessory factor in this elaborate assembly pathway. However, the structural location of Psb27 within PSII assembly intermediates has remained elusive. Here we report that Psb27 binds to CP43 in such assembly intermediates. We treated purified genetically tagged PSII assembly intermediate complexes from the cyanobacterium Synechocystis 6803 with chemical cross-linkers to examine intermolecular interactions between Psb27 and various PSII proteins. First, the water-soluble 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) was used to cross-link proteins with complementary charged groups in close association to one another. In the His27△ctpAPSII preparation, a 58-kDa cross-linked species containing Psb27 and CP43 was identified. This species was not formed in the HT3△ctpA△psb27PSII complex in which Psb27 was absent. Second, the homobifunctional thiol-cleavable cross-linker 3,3'-dithiobis(sulfosuccinimidylpropionate) (DTSSP) was used to reversibly cross-link Psb27 to CP43 in His27△ctpAPSII preparations, which allowed the use of liquid chromatography/tandem MS to map the cross-linking sites as Psb27K(63)↔CP43D(321) (trypsin) and CP43K(215)↔Psb27D(58)AGGLK(63)↔CP43D(321) (chymotrypsin), respectively. Our data suggest that Psb27 acts as an important regulatory protein during PSII assembly through specific interactions with the luminal domain of CP43.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Umena Y, Kawakami K, Shen JR, Kamiya N. Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å. Nature. 2011;473:55–60. - PubMed
-
- Roose JL, Pakrasi HB. The Psb27 protein facilitates manganese cluster assembly in photosystem II. J Biol Chem. 2008;283:4044–4050. - PubMed
-
- Ikeuchi M, Inoue Y, Vermaas W. Characterization of photosystem II subunits from the cyanobacterium Synechocystis sp. PCC6803. In: Mathis P, editor. Photosynthesis: From Light to Biosphere. Vol III. Dordrecht, The Netherlands: Kluwer; 1995. pp. 297–300.
-
- Kashino Y, et al. Proteomic analysis of a highly active photosystem II preparation from the cyanobacterium Synechocystis sp. PCC 6803 reveals the presence of novel polypeptides. Biochemistry. 2002;41:8004–8012. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

