Activation mechanism of the β2-adrenergic receptor
- PMID: 22031696
- PMCID: PMC3219117
- DOI: 10.1073/pnas.1110499108
Activation mechanism of the β2-adrenergic receptor
Abstract
A third of marketed drugs act by binding to a G-protein-coupled receptor (GPCR) and either triggering or preventing receptor activation. Although recent crystal structures have provided snapshots of both active and inactive functional states of GPCRs, these structures do not reveal the mechanism by which GPCRs transition between these states. Here we propose an activation mechanism for the β(2)-adrenergic receptor, a prototypical GPCR, based on atomic-level simulations in which an agonist-bound receptor transitions spontaneously from the active to the inactive crystallographically observed conformation. A loosely coupled allosteric network, comprising three regions that can each switch individually between multiple distinct conformations, links small perturbations at the extracellular drug-binding site to large conformational changes at the intracellular G-protein-binding site. Our simulations also exhibit an intermediate that may represent a receptor conformation to which a G protein binds during activation, and suggest that the first structural changes during receptor activation often take place on the intracellular side of the receptor, far from the drug-binding site. By capturing this fundamental signaling process in atomic detail, our results may provide a foundation for the design of drugs that control receptor signaling more precisely by stabilizing specific receptor conformations.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
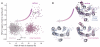

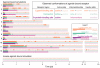
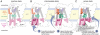
References
-
- Rasmussen SGF, et al. Crystal structure of the β2 adrenergic receptor-Gs protein complex. Nature. 2011 10.1038/nature10361. - DOI - PMC - PubMed
-
- Palczewski K, et al. Crystal structure of rhodopsin: A G protein-coupled receptor. Science. 2000;289:739–745. - PubMed
-
- Choe HW, et al. Crystal structure of metarhodopsin II. Nature. 2011;471:651–655. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

