Coordinated regulation of lymph node vascular-stromal growth first by CD11c+ cells and then by T and B cells
- PMID: 22031764
- PMCID: PMC3221869
- DOI: 10.4049/jimmunol.1101724
Coordinated regulation of lymph node vascular-stromal growth first by CD11c+ cells and then by T and B cells
Abstract
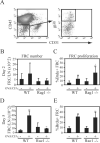
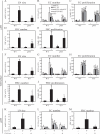
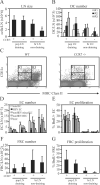
Lymph node blood vessels play important roles in the support and trafficking of immune cells. The blood vasculature is a component of the vascular-stromal compartment that also includes the lymphatic vasculature and fibroblastic reticular cells (FRCs). During immune responses as lymph nodes swell, the blood vasculature undergoes a rapid proliferative growth that is initially dependent on CD11c(+) cells and vascular endothelial growth factor (VEGF) but is independent of lymphocytes. The lymphatic vasculature grows with similar kinetics and VEGF dependence, suggesting coregulation of blood and lymphatic vascular growth, but lymphatic growth has been shown to be B cell dependent. In this article, we show that blood vascular, lymphatic, and FRC growth are coordinately regulated and identify two distinct phases of vascular-stromal growth--an initiation phase, characterized by upregulated vascular-stromal proliferation, and a subsequent expansion phase. The initiation phase is CD11c(+) cell dependent and T/B cell independent, whereas the expansion phase is dependent on B and T cells together. Using CCR7(-/-) mice and selective depletion of migratory skin dendritic cells, we show that endogenous skin-derived dendritic cells are not important during the initiation phase and uncover a modest regulatory role for CCR7. Finally, we show that FRC VEGF expression is upregulated during initiation and that dendritic cells can stimulate increased fibroblastic VEGF, suggesting the scenario that lymph node-resident CD11c(+) cells orchestrate the initiation of blood and lymphatic vascular growth in part by stimulating FRCs to upregulate VEGF. These results illustrate how the lymph node microenvironment is shaped by the cells it supports.
Figures




References
-
- von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat Rev Immunol. 2003;3 - PubMed
-
- Lammermann T, Sixt M. The microanatomy of T-cell responses. Immunol Rev. 2008;221 - PubMed
-
- Junt T, Scandella E, Ludewig B. Form follows function: lymphoid tissue microarchitecture in antimicrobial immune defence. Nat Rev Immunol. 2008;8 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

