Antiviral activity and increased host defense against influenza infection elicited by the human cathelicidin LL-37
- PMID: 22031815
- PMCID: PMC3198734
- DOI: 10.1371/journal.pone.0025333
Antiviral activity and increased host defense against influenza infection elicited by the human cathelicidin LL-37
Abstract
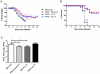
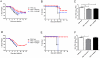
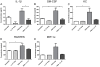
The extensive world-wide morbidity and mortality caused by influenza A viruses highlights the need for new insights into the host immune response and novel treatment approaches. Cationic Host Defense Peptides (CHDP, also known as antimicrobial peptides), which include cathelicidins and defensins, are key components of the innate immune system that are upregulated during infection and inflammation. Cathelicidins have immunomodulatory and anti-viral effects, but their impact on influenza virus infection has not been previously assessed. We therefore evaluated the effect of cathelicidin peptides on disease caused by influenza A virus in mice. The human cathelicidin, LL-37, and the murine cathelicidin, mCRAMP, demonstrated significant anti-viral activity in vivo, reducing disease severity and viral replication in infected mice to a similar extent as the well-characterized influenza virus-specific antiviral drug zanamivir. In vitro and in vivo experiments suggested that the peptides may act directly on the influenza virion rather than via receptor-based mechanisms. Influenza virus-infected mice treated with LL-37 had lower concentrations of pro-inflammatory cytokines in the lung than did infected animals that had not been treated with cathelicidin peptides. These data suggest that treatment of influenza-infected individuals with cathelicidin-derived therapeutics, or modulation of endogenous cathelicidin production may provide significant protection against disease.
Conflict of interest statement
Figures


References
-
- Wu S, Metcalf JP, Wu W. Innate immune response to influenza virus. Curr Opin Infect Dis. 2011. - PubMed
-
- Salvatore M, Garcia-Sastre A, Ruchala P, Lehrer RI, Chang T, et al. alpha -Defensin Inhibits Influenza Virus Replication by Cell-Mediated Mechanism(s). J Infect Dis. 2007;196:835–843. - PubMed
-
- Tecle T, White MR, Gantz D, Crouch EC, Hartshorn KL. Human neutrophil defensins increase neutrophil uptake of influenza A virus and bacteria and modify virus-induced respiratory burst responses. J Immunol. 2007;178:8046–8052. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

