Monocyte chemoattractant protein-1-deficiency impairs the expression of IL-6, IL-1β and G-CSF after transient focal ischemia in mice
- PMID: 22031820
- PMCID: PMC3198727
- DOI: 10.1371/journal.pone.0025863
Monocyte chemoattractant protein-1-deficiency impairs the expression of IL-6, IL-1β and G-CSF after transient focal ischemia in mice
Abstract
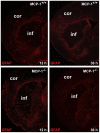
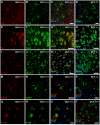
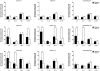
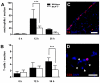
Monocyte chemoattractant protein-1 (MCP-1), a chemokine secreted by neurons and astrocytes following stroke is known to aggravate ischemia-related damage. Previous studies revealed that MCP-1-deficient mice develop smaller infarcts and have an improved neurological outcome, whereas mice overexpressing MCP-1 show worsened brain damage and impaired neurological function. The aim of the present study was to elucidate the molecular background of the enhanced recovery in MCP-1-deficient mice after stroke. For this purpose, we (1) performed expression analyses on crucial post-stroke related inflammatory genes in MCP-1-deficient mice compared to wildtype controls, (2) analyzed a possible impact of MCP-1 on astrocyte activation (3) investigated the cellular origin of respective inflammatory cytokines and (4) analyzed the impact of MCP-1 secretion on the migration of both neutrophil granulocytes and T-cells. Here we report that MCP-1-deficiency leads to a shift towards a less inflammatory state following experimental occlusion of the middle cerebral artery including an impaired induction of interleukin-6, interleukin-1β and granulocyte-colony stimulating factor expression as well as a subsequent diminished influx of hematogenous cells. Additionally, MCP-1-deficient mice developed smaller infarcts 36 hours after experimental stroke. Investigations revealed no differences in transcription of tumor necrosis factor-α and astrogliosis 12 and 36 hours after onset of ischemia. These novel results help to understand post ischemic, inflammatory mechanisms and might give further arguments towards therapeutical interventions by modulation of MCP-1 expression in post stroke inflammation.
Conflict of interest statement
Figures


Similar articles
-
Monocyte chemoattractant protein-1-deficiency results in altered blood-brain barrier breakdown after experimental stroke.Stroke. 2013 Sep;44(9):2536-44. doi: 10.1161/STROKEAHA.111.000528. Epub 2013 Jul 2. Stroke. 2013. PMID: 23821228
-
Effects of monocyte chemoattractant protein 1 on blood-borne cell recruitment after transient focal cerebral ischemia in mice.Neuroscience. 2009 Jul 7;161(3):806-12. doi: 10.1016/j.neuroscience.2009.04.025. Epub 2009 Apr 15. Neuroscience. 2009. PMID: 19374937
-
Monocyte chemoattractant protein-1 expressed in neurons and astrocytes during focal ischemia in mice.Brain Res. 2001 Jun 1;902(2):171-7. doi: 10.1016/s0006-8993(01)02328-9. Brain Res. 2001. PMID: 11384610
-
MCP-1/CCR-2-double-deficiency severely impairs the migration of hematogenous inflammatory cells following transient cerebral ischemia in mice.Exp Neurol. 2012 Feb;233(2):849-58. doi: 10.1016/j.expneurol.2011.12.011. Epub 2011 Dec 16. Exp Neurol. 2012. PMID: 22197827
-
Monocyte chemoattractant protein-1 deficiency is protective in a murine stroke model.J Cereb Blood Flow Metab. 2002 Mar;22(3):308-17. doi: 10.1097/00004647-200203000-00008. J Cereb Blood Flow Metab. 2002. PMID: 11891436
Cited by
-
Protective Effects of Cannabidivarin and Cannabigerol on Cells of the Blood-Brain Barrier Under Ischemic Conditions.Cannabis Cannabinoid Res. 2021 Aug;6(4):315-326. doi: 10.1089/can.2020.0159. Epub 2021 Mar 17. Cannabis Cannabinoid Res. 2021. PMID: 33998890 Free PMC article.
-
Monocyte chemotactic protein-1 as a potential biomarker for early anti-thrombotic therapy after ischemic stroke.Int J Mol Sci. 2012;13(7):8670-8678. doi: 10.3390/ijms13078670. Epub 2012 Jul 12. Int J Mol Sci. 2012. PMID: 22942727 Free PMC article. Clinical Trial.
-
Elevated monocyte-to-HDL cholesterol ratio predicts post-stroke depression.Front Psychiatry. 2022 Jul 22;13:902022. doi: 10.3389/fpsyt.2022.902022. eCollection 2022. Front Psychiatry. 2022. PMID: 35935403 Free PMC article.
-
Lack of collagen XV is protective after ischemic stroke in mice.Cell Death Dis. 2017 Jan 12;8(1):e2541. doi: 10.1038/cddis.2016.456. Cell Death Dis. 2017. PMID: 28079884 Free PMC article.
-
Interaction of coexposure to inorganic arsenic and manganese: Tight junction injury of the blood-brain barrier and the relationship between oxidative stress and inflammatory cytokines in glial cells.PLoS One. 2025 Aug 25;20(8):e0330287. doi: 10.1371/journal.pone.0330287. eCollection 2025. PLoS One. 2025. PMID: 40853884 Free PMC article.
References
-
- Lin N, Friedlander RM. T-cell activation in ischemic stroke: a new therapeutic target for delayed infarct expansion? Neurosurgery. 2009;65(6):N13. - PubMed
-
- Kriz J, Lalancette-Hébert M. Inflammation, plasticity and real-time imaging after cerebral ischemia. Acta Neuropathol. 2009;117(5):497–509. - PubMed
-
- Gelderblom M, Leypoldt F, Steinbach K, Behrens D, Choe CU, et al. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke. 2009;40(5):1849–57. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

