Recent perspectives into biochemistry of decavanadate
- PMID: 22031844
- PMCID: PMC3202125
- DOI: 10.4331/wjbc.v2.i10.215
Recent perspectives into biochemistry of decavanadate
Abstract
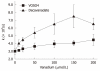
The number of papers about decavanadate has doubled in the past decade. In the present review, new insights into decavanadate biochemistry, cell biology, and antidiabetic and antitumor activities are described. Decameric vanadate species (V(10)) clearly differs from monomeric vanadate (V(1)), and affects differently calcium pumps, and structure and function of myosin and actin. Only decavanadate inhibits calcium accumulation by calcium pump ATPase, and strongly inhibits actomyosin ATPase activity (IC(50) = 1.4 μmol/L, V(10)), whereas no such effects are detected with V(1) up to 150 μmol/L; prevents actin polymerization (IC(50) of 68 μmol/L, whereas no effects detected with up to 2 mmol/L V(1)); and interacts with actin in a way that induces cysteine oxidation and vanadate reduction to vanadyl. Moreover, in vivo decavanadate toxicity studies have revealed that acute exposure to polyoxovanadate induces different changes in antioxidant enzymes and oxidative stress parameters, in comparison with vanadate. In vitro studies have clearly demonstrated that mitochondrial oxygen consumption is strongly affected by decavanadate (IC(50), 0.1 μmol/L); perhaps the most relevant biological effect. Finally, decavanadate (100 μmol/L) increases rat adipocyte glucose accumulation more potently than several vanadium complexes. Preliminary studies suggest that decavanadate does not have similar effects in human adipocytes. Although decavanadate can be a useful biochemical tool, further studies must be carried out before it can be confirmed that decavanadate and its complexes can be used as anticancer or antidiabetic agents.
Keywords: Actin; Actin polymerization; Antidiabetic agent; Antitumor agent; Calcium pump; Decavanadate; Insulin mimetic; Myosin; Vanadate.
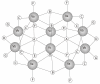
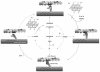
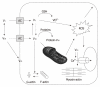
Figures


References
-
- Nriagu JO. Vanadium in the Environment. In: Adv Environ., editor. Science Technology. Hoboken: John Wiley & Sons; 1998. p. Parts 1 and 2.
-
- Ashok K. Vanadium Compounds: Biochemical and Therapeutic Applications (Developments in Molecular and Cellular Biochemistry). Srivastava, Chiasson JL, editors. New York: Springer; 1996.
-
- Tracey AS, Willsky GR, Takeuschi ES. Vanadium. Chemistry, Biochemistry, Pharmacology and Practical Applications. Boca Raton: CRC Press, Taylor & Francis Group; 2007.
-
- Kustin K, Costa Pessoa J, Crans DC. Vanadium the Versatile Metal, ACS Symposium Series. Washington DC: Oxford University Press; 2007.
-
- Aureliano M. Vanadium biochemistry. Kerala, India: Research Signpost Publishers; 2007.
LinkOut - more resources
Full Text Sources
Miscellaneous

