The selective androgen receptor modulator GTx-024 (enobosarm) improves lean body mass and physical function in healthy elderly men and postmenopausal women: results of a double-blind, placebo-controlled phase II trial
- PMID: 22031847
- PMCID: PMC3177038
- DOI: 10.1007/s13539-011-0034-6
The selective androgen receptor modulator GTx-024 (enobosarm) improves lean body mass and physical function in healthy elderly men and postmenopausal women: results of a double-blind, placebo-controlled phase II trial
Abstract
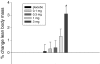
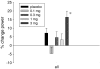
BACKGROUND: Cachexia, also known as muscle wasting, is a complex metabolic condition characterized by loss of skeletal muscle and a decline in physical function. Muscle wasting is associated with cancer, sarcopenia, chronic obstructive pulmonary disease, end-stage renal disease, and other chronic conditions and results in significant morbidity and mortality. GTx-024 (enobosarm) is a nonsteroidal selective androgen receptor modulator (SARM) that has tissue-selective anabolic effects in muscle and bone, while sparing other androgenic tissue related to hair growth in women and prostate effects in men. GTx-024 has demonstrated promising pharmacologic effects in preclinical studies and favorable safety and pharmacokinetic profiles in phase I investigation. METHODS: A 12-week double-blind, placebo-controlled phase II clinical trial was conducted to evaluate GTx-024 in 120 healthy elderly men (>60 years of age) and postmenopausal women. The primary endpoint was total lean body mass assessed by dual energy X-ray absorptiometry, and secondary endpoints included physical function, body weight, insulin resistance, and safety. RESULTS: GTx-024 treatment resulted in dose-dependent increases in total lean body mass that were statistically significant (P < 0.001, 3 mg vs. placebo) and clinically meaningful. There were also significant improvements in physical function (P = 0.013, 3 mg vs. placebo) and insulin resistance (P = 0.013, 3 mg vs. placebo). The incidence of adverse events was similar between treatment groups. CONCLUSION: GTx-024 showed a dose-dependent improvement in total lean body mass and physical function and was well tolerated. GTx-024 may be useful in the prevention and/or treatment of muscle wasting associated with cancer and other chronic diseases.
Figures
References
-
- Roubenoff R. Sarcopenia and its implications for the elderly. Eur J Clin Nutr. 2000;54(Suppl 3):S40–S47. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

