Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes
- PMID: 22031862
- PMCID: PMC3265200
- DOI: 10.1182/blood-2011-02-338004
Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes
Abstract
Dendritic cells (DCs) are the most potent APCs. Whereas immature DCs down-regulate T-cell responses to induce/maintain immunologic tolerance, mature DCs promote immunity. To amplify their functions, DCs communicate with neighboring DCs through soluble mediators, cell-to-cell contact, and vesicle exchange. Transfer of nanovesicles (< 100 nm) derived from the endocytic pathway (termed exosomes) represents a novel mechanism of DC-to-DC communication. The facts that exosomes contain exosome-shuttle miRNAs and DC functions can be regulated by exogenous miRNAs, suggest that DC-to-DC interactions could be mediated through exosome-shuttle miRNAs, a hypothesis that remains to be tested. Importantly, the mechanism of transfer of exosome-shuttle miRNAs from the exosome lumen to the cytosol of target cells is unknown. Here, we demonstrate that DCs release exosomes with different miRNAs depending on the maturation of the DCs. By visualizing spontaneous transfer of exosomes between DCs, we demonstrate that exosomes fused with the target DCs, the latter followed by release of the exosome content into the DC cytosol. Importantly, exosome-shuttle miRNAs are functional, because they repress target mRNAs of acceptor DCs. Our findings unveil a mechanism of transfer of exosome-shuttle miRNAs between DCs and its role as a means of communication and posttranscriptional regulation between DCs.
Figures


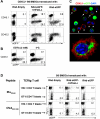

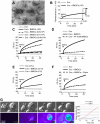
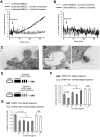
Comment in
-
Functional transfer of microRNA by exosomes.Blood. 2012 Jan 19;119(3):646-8. doi: 10.1182/blood-2011-11-389478. Blood. 2012. PMID: 22262739 No abstract available.
-
Programmed vesicle transfer of green fluorescent protein from a stably transduced cell line to primary hematopoietic cells.Blood. 2012 May 31;119(22):5330-2. doi: 10.1182/blood-2012-04-422477. Blood. 2012. PMID: 22653957 No abstract available.
References
-
- Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(7):654–659. - PubMed
-
- Taylor DD, Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol. 2008;110(1):13–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

